Abstract
All cells regulate their intracellular zinc levels. In yeast, zinc uptake is mediated by Zrt1p and Zrt2p, which belong to the ZIP family of metal transporters. Under zinc limitation, ZRT1 and ZRT2 transcription is induced by the Zap1p transcriptional activator. We describe here a new component of zinc homeostasis, vacuolar zinc storage, that is also regulated by Zap1p. Zinc-replete cells accumulate zinc in the vacuole via the Zrc1p and Cot1p transporters. Our results indicate that another zinc transporter, Zrt3p, mobilizes this stored zinc in zinc-limited cells. ZRT3 is a Zap1p-regulated gene whose transcription increases in low zinc. Zrt3p is also a member of the ZIP family and it localizes to the vacuolar membrane. The effects of ZRT3 mutation and overexpression on cell growth, cellular zinc accumulation and intracellular labile zinc pools are all consistent with its proposed role. Furthermore, we demonstrate that zrt3 mutants inefficiently mobilize stored zinc to offset deficiency. Thus, our studies define a system of zinc influx and efflux transporters in the vacuole that play important roles in zinc homeostasis.
Keywords: homeostasis/storage/transporter/vacuole/zinc
Introduction
Zinc is an essential nutrient for life. As a structural component of the zinc finger motifs found in many transcription factors and as a catalytic co-factor for RNA polymerase, zinc is required for gene transcription (Vallee and Falchuk, 1993). Zinc is also a cofactor of many essential enzymes. Consistent with the importance of zinc to cellular metabolism, zinc deficiency can have severe consequences. In mammals, for example, even moderate zinc deficiency can cause anemia, loss of appetite, immune system defects, developmental problems and teratogenesis. However, excess zinc can also be detrimental to cells. For this reason, the cytoplasmic concentration of loosely bound, labile zinc must be tightly controlled. This homeostatic regulation is mediated through a variety of mechanisms, including the control of zinc uptake (Guerinot and Eide, 1999), intracellular binding of the metal by metallothioneins (Palmiter, 1998) and zinc efflux from the cell (Nies and Silver, 1995; Palmiter and Findley, 1995). In this report, we describe the transporters involved in the regulated storage of zinc in an intracellular organelle.
Studies of the yeast Saccharomyces cerevisiae have led to rapid progress in understanding zinc homeostasis at the molecular level (Guerinot and Eide, 1999). Zinc uptake in S.cerevisiae is mediated primarily by two different systems in the plasma membrane: a high affinity system encoded by ZRT1 (Zhao and Eide, 1996a) and a lower affinity system encoded by ZRT2 (Zhao and Eide, 1996b). Zrt1p and Zrt2p are related proteins belonging to the ‘ZIP’ superfamily, whose members are found in many different eukaryotic organisms (Eng et al., 1998). Other characterized members of this family also play roles in metal ion transport. Zip1p–Zip4p and Irt1p of Arabidopsis transport zinc (and in the case of Irt1p, iron and manganese) across the membranes of plant cells (Grotz et al., 1998; Korshunova et al., 1999). Furthermore, two human ZIPs, hZip1 and hZip2, have recently been implicated in mammalian zinc transport (Costello et al., 1999; Gaither and Eide, 2000).
Expression of both ZRT1 and ZRT2 increases in zinc-limited cells (Zhao and Eide, 1996a,b). This induction is mediated by the Zap1p transcriptional activator, which also up-regulates its own expression under zinc limitation (Zhao and Eide, 1997). The activity of Zap1p is repressed in zinc-replete cells. While the mechanism of this regulation is unknown, Zap1p is likely to sense the concentration of a labile pool of cytoplasmic/nuclear zinc. Zap1p binds to a conserved 11 bp sequence, called the zinc-responsive element (ZRE), present in the ZRT1, ZRT2 and ZAP1 promoters (Zhao et al., 1998). A consensus sequence derived from the ZREs in these promoters is 5′-ACCYYNAAGGT-3′. One ZRE, in either orientation relative to the gene, is sufficient to confer Zap1p-regulated expression on a promoter.
Another potential participant in yeast zinc homeostasis is the vacuole. The vacuole accumulates several divalent cations, including Ca2+, Co2+, Fe2+, Mn2+ and Ni2+ (Dunn et al., 1994; Bode et al., 1995; Ramsay and Gadd, 1997; Nishimura et al., 1998; Paidhungat and Garrett, 1998). This compartment has also been implicated as a site of zinc detoxification. For example, increased zinc sensitivity has been observed in mutants defective in either vacuole biogenesis or acidification (Eide et al., 1993; Ramsay and Gadd, 1997). The requirement for vacuole acidification suggests that the accumulation of zinc may be coupled to the discharge of the proton gradient across the vacuolar membrane. Two proteins implicated in transporting zinc into the vacuole are Zrc1p and Cot1p. While Cot1p was originally thought to be in mitochondria (Conklin et al., 1992), more recent studies have established that both it and Zrc1p are located predominantly in the vacuolar membrane (Li and Kaplan, 1998). Overexpression of either leads to increased zinc resistance, while mutation of each gene decreases tolerance (Kamizono et al., 1989; Conklin et al., 1992, 1994). Zrc1p and Cot1p are both members of the CDF family of transporters (Paulsen and Saier, 1997), which also includes the mammalian ZnT proteins. ZnT-1 is a probable zinc transporter responsible for efflux across the plasma membrane (Palmiter and Findley, 1995), whereas ZnT-2 transports zinc into late endosomes (Palmiter et al., 1996a; Kobayashi et al., 1999). ZnT-3 localizes to synaptic vesicles and facilitates transport of zinc into this compartment for release with neurotransmitters (Palmiter et al., 1996b). ZnT-4 is expressed in intestinal cells (Murgia et al., 1999) and the mammary gland where it is required for zinc transport into milk (Huang and Gitschier, 1997). Given the similar function of these diverse family members, it is likely that Zrc1p and Cot1p mediate zinc transport into the vacuole.
To identify additional Zap1p-regulated genes involved in yeast zinc homeostasis, we scanned the complete genome sequence for genes with promoters that contain potential ZREs. Our characterization of one such gene, YKL175w (which we have designated ZRT3 for zinc regulated transporter 3), indicates that this gene encodes a new member of the ZIP transporter family. Zrt3p’s apparent function is to transport stored zinc out of the vacuole during the transition from zinc-replete to zinc-limiting conditions. These studies demonstrate the importance of organellar zinc storage and a new role for ZIP transporters in eukaryotic cells. Furthermore, the identification of Zrt3p as a ZIP transporter allows the extension of this family, formerly limited to eukaryotes, to include previously unknown members from the archaea and eubacteria.
Results
Identification of the ZRT3 gene
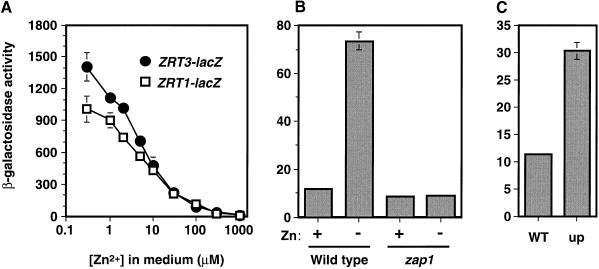
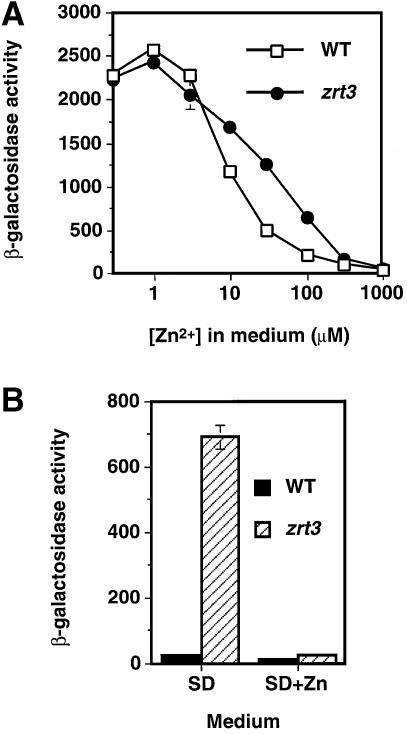
Thirty genes in the yeast genome contain one or more consensus ZRE within 1000 bp upstream of their predicted ATG start codons. One of these genes, YKL175w (now called ZRT3), was chosen for further analysis because of its similarity to ZIP transporters (see below). A single potential ZRE (ACCCTTAAGGT) is located in the ZRT3 promoter from –145 to –155 bp upstream of the open reading frame. To test whether ZRT3 is regulated by Zap1p, the ZRT3 promoter was fused to the Escherichia coli lacZ gene. Analysis of β-galactosidase activity in a wild-type strain bearing this reporter showed that the ZRT3 promoter was highly expressed in zinc-limited cells, but not in zinc-replete cells (Figure 1A). Moreover, the ZRT3–lacZ expression profile was almost identical to that observed with a ZRT1–lacZ promoter fusion over a wide range of zinc concentrations. Analysis of ZRT3–lacZ expression in strains bearing mutations in the ZAP1 gene further indicated that ZRT3 induction was dependent on Zap1p; a zap1 mutant did not induce ZRT3–lacZ activity under zinc limitation (Figure 1B). Moreover, a strain carrying the ZAP1-1up mutation, an allele that increases expression of Zap1p-regulated genes in zinc-replete cells (Zhao and Eide, 1997), showed an increased level of ZRT3–lacZ expression (Figure 1C). These results demonstrate that the ZRT3 gene is a target for Zap1p regulation.
Fig. 1. ZRT3 is a Zap1p target gene. (A) Wild-type (DY1457) cells bearing ZRT1–lacZ (pGI3) or ZRT3–lacZ (pAG3) reporters were grown in LZM supplemented with the indicated concentration of ZnCl2 and assayed for β-galactosidase activity. (B) Wild-type (DY1457) and zap1 mutant (ZHY6) cells bearing pAG3 were grown in high (+; SD supplemented with 2 µM ZnCl2) or low (–; SD supplemented with 1 mM EDTA, 50 µM ZnCl2) zinc medium. (C) Wild type (DY1457) and ZAP1-1up mutants (ZHY7; up) bearing pAG3 were grown in a zinc-replete medium (SD + 2 µM ZnCl2). One representative experiment is shown in each panel; data are the means of three replicates and the error bars represent ± 1 SD.
Zrt3p is a member of the ZIP transporter family
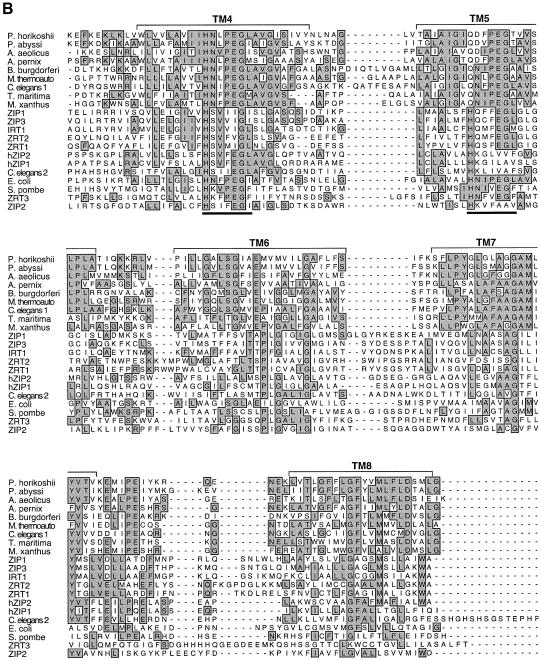
The ZRT3 gene encodes a protein with a predicted molecular mass of 55 kDa (Figure 2A). While Zrt3p is not closely related to Zrt1p and Zrt2p, a number of observations led us to conclude that Zrt3p is also a member of the ZIP family. First, both Zrt3p and most known ZIP transporters contain eight predicted transmembrane (TM) domains (Eng et al., 1998). Secondly, many of the ZIP proteins have a long loop region, termed the ‘variable region’, between TM3 and TM4 (Grotz et al., 1998). Zrt3p also shares this feature. Furthermore, in most of the ZIPs, the variable region contains the repeat sequence (HX)n (where n ≥ 3), as does Zrt3p. While the function of this motif is unknown, its presence in Zrt3p provides an additional link between this protein and other ZIP family transporters. Clusters of histidines are also found elsewhere in the variable region and in one other location, between TM7 and TM8, in Zrt3p.

Fig. 2. ZRT3 encodes a member of the ZIP superfamily. (A) The amino acid sequence of Zrt3p, with predicted transmembrane domains shaded and clusters of histidines underlined. (B) Sequence similarity of Zrt3p to ZIP family members and newly identified proteins. The segments containing predicted transmembrane domains 4–8 of these proteins are shown. Boxed and shaded residues match the consensus identity at each position, the amphipathic regions of TM4 and TM5 are underlined. The DDBJ/EMBL/GenBank protein sequence accession numbers for the new ZIP family members are: C.elegans 1, AAB37816; S.pombe, CAA22478; M.thermoautotrophicum, AAB84979; E.coli, P24198; P.horikoshii, BAA29722; Myxococcus xanthus, Q06916; B.burgdorferi, AAC66605; Thermotoga maritima, AAD36803; Pyrococcus abyssi, CAB50314; Aeropyrum pernix, BAA80692; Aquifex aeolicus, AAC07647; ZRT3, CAA82017. Accession numbers for previously described ZIP family members are: ZRT1, CAA96975.1; ZRT2, 6323159; ZIP1, AAC24197; ZIP2, AAC24198; ZIP3, AAC24199; hZIP1, AAD27717; hZIP2, AAB34328; C.elegans 2, AAB37814. The last 11 residues of the C.elegans 2 sequence are not shown.
Database searches indicated that Zrt3p is related to some previously identified ZIP family members (e.g. AAB37814 of Caenorhabditis elegans). The greatest sequence conservation among all of the members of the ZIP family, and their similarity with Zrt3p, extends from TM4 through TM8 (Figure 2B). A specific feature shared among the ZIPs is the amphipathic nature of the TM4 and TM5 helices, and this property is also true of Zrt3p. This amphipathicity suggested that TM4 and TM5 may in part comprise the transmembrane aqueous channel through which metal ions travel (Eng et al., 1998). Within TM4, the ZIPs have an invariant histidine followed by an adjacent polar or charged residue. These residues are conserved in Zrt3p. Similarly, TM5 of Zrt3p and the ZIPs contain a largely conserved histidine and nearby glutamate that are also essential for Irt1p function. Thus, based on shared protein topology and sequence similarity, as well as the conservation of presumably critical residues in TM4 and TM5, we conclude that Zrt3p is a bona fide member of the ZIP family of transporters.
Database searches using the Zrt3p sequence also identified a number of closely related gene products of unknown function and several are included in the alignment shown in Figure 2B. These proteins derive from the archaea (e.g. Methanobacterium thermoautotrophicum, Pyrococcus horikoshii) and eubacteria (e.g. Borrelia burgdorferi, E.coli) as well as other eukaryotes (e.g. Schizosaccharomyces pombe). Thus, Zrt3p provides a link between the previously recognized eukaryotic ZIPs and their prokaryotic and more distant eukaryotic relatives.
Effect of the zrt3 mutation on cell growth
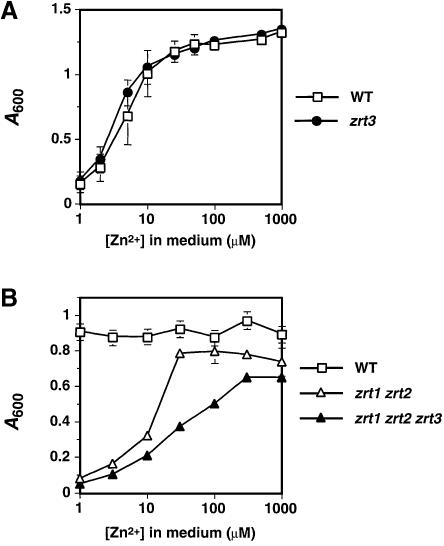
Recognition of Zrt3p as a ZIP family member suggested that this protein might be a zinc transporter. To determine its function, the ZRT3 gene was deleted in a haploid yeast strain. The resulting zrt3 mutant was viable in both rich (YPD) and minimal synthetic defined (SD) media, indicating that Zrt3p is not required for growth under standard laboratory conditions. To test whether Zrt3p is important under zinc limitation, growth of wild-type and zrt3 mutant strains was examined in a low zinc medium (LZM) supplemented with increasing concentrations of zinc (Figure 3A). No difference in growth yield was observed between these two strains, indicating that Zrt3p is not required in low or replete zinc conditions.
Fig. 3. Effect of the zrt3 mutation on zinc-limited growth. (A) Wild-type (DY1457) and zrt3 mutant (CMK1) strains were grown to stationary phase in SD medium and inoculated into LZM supplemented with the indicated concentration of ZnCl2 at a starting A600 of 0.01. After 20 h incubation at 30°C, the culture A600 values were determined; the values are the means of three independent experiments and the error bars represent ± 1 SD. (B) Growth of wild-type (CM30), zrt1 zrt2 (CM34) and zrt1 zrt2 zrt3 (CM37) strains in LZM-EDTA with the indicated concentrations of zinc added. Otherwise, the experiment was performed as described for (A). The means of two independent experiments are shown and the error bars represent ± 1 SD.
Because Zrt3p is related to the Zrt1p and Zrt2p zinc uptake transporters, we suspected that these proteins might play overlapping roles. To test this hypothesis, we compared growth of a zrt1 zrt2 double mutant to that of an isogenic zrt1 zrt2 zrt3 triple mutant over a range of zinc availability. LZM is zinc limiting because it contains EDTA, a high affinity zinc chelator. Because zrt1 zrt2 mutants have impaired zinc uptake, their zinc requirement was assessed in LZM prepared without EDTA (LZM-EDTA). This medium is less zinc limiting than LZM at a given concentration of total zinc because citrate, the predominant chelator in LZM-EDTA, binds the metal with lower affinity than EDTA. While the zrt1 zrt2 mutant reached its maximum growth yield at 50 µM total zinc, the zrt1 zrt2 zrt3 mutant required 10-fold more zinc (500 µM) to attain its maximum yield (Figure 3B). The increased zinc requirement of the triple mutant suggests that these three genes overlap in function, and that Zrt3p also transports Zn2+ into the cytoplasm where it is available for use in zinc-dependent processes.
Effects of ZRT3 mutation and overexpression on zinc accumulation
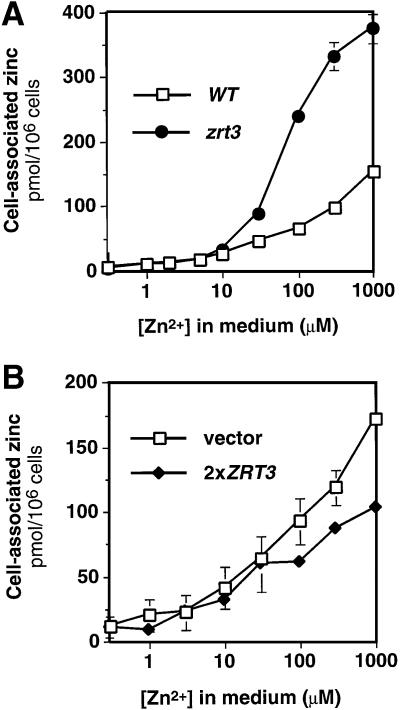
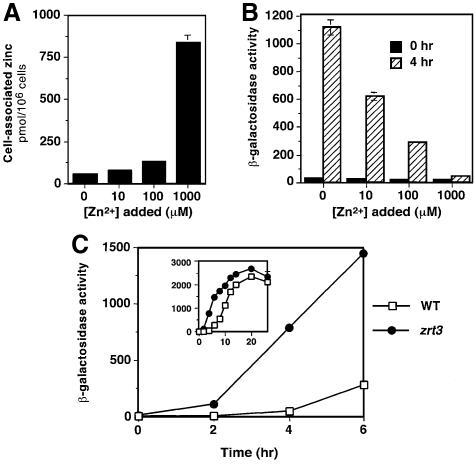
If Zrt3p mediates zinc uptake across the plasma membrane, mutation of this gene would impair zinc accumulation, as do the zrt1 and zrt2 mutations (Zhao and Eide, 1996a,b). To test this prediction, we measured the total cell-associated zinc content of wild-type and zrt3 mutant cells after growth in zinc-limited medium supplemented with a range of zinc concentrations (Figure 4A). Surprisingly, rather than a decrease, the zrt3 mutant showed a marked increase in total cellular zinc when grown in LZM containing 10–1000 µM zinc. No difference between the strains was seen at lower zinc concentrations (0.3–3 µM zinc in LZM). While the ZRT3–lacZ fusion results argue that ZRT3 expression is low in zinc-replete cells (Figure 1A), the effects of the zrt3 mutation on zinc accumulation (Figure 4A) show that the gene is expressed at a significant level in these cells.
Fig. 4. Effects of zrt3 mutation and ZRT3 overexpression on cellular zinc accumulation. (A) Wild-type (DY1457) and zrt3 mutant (CMK1) strains were grown to exponential phase in LZM supplemented with the indicated concentration of ZnCl2. Cells were harvested and assayed for cell-associated zinc levels by atomic absorption spectroscopy (AAS). (B) Wild-type cells (DY1457) transformed with a centromeric plasmid containing the ZRT3 gene (pCM2) or the vector alone (pFL39) were assayed as described for (A). For (A) and (B), values are the means of three independent experiments and the error bars indicate ± 1 SD.
We also determined the effect of ZRT3 overexpression on zinc accumulation. High level overexpression of ZRT3 from the galactose-inducible GAL1 promoter or a high copy plasmid vector resulted in poor growth. This effect was not suppressed by either zinc supplements or limitation, suggesting that the phenotype may result from fortuitous toxicity. When a single-copy plasmid clone of the wild-type ZRT3 gene was introduced into a wild-type strain, potentially resulting in overexpression due to a doubling of gene dosage, no growth defect was observed. When these cells were grown in LZM with added zinc, a significant decrease in zinc accumulation was observed relative to the vector-only control (Figure 4B). Again, these effects were only observed at higher zinc concentrations. Given the unexpected effects of zrt3 mutation and overexpression on zinc accumulation, these results demonstrate that Zrt3p clearly plays a different role in zinc metabolism than Zrt1p and Zrt2p. Paradoxically, the results are consistent with a role for Zrt3p in decreasing cellular zinc accumulation.
Effect of the ZRT3 gene on the regulatory zinc pool
If the excess zinc accumulated in zrt3 mutants was distributed throughout the cell, an increase in the labile pool of cytoplasmic/nuclear zinc sensed by Zap1p might be expected. Although we cannot measure this zinc pool directly, a comparative estimate can be obtained by quantifying Zap1p activity using a ZRE–lacZ reporter (Zhao and Eide, 1996a,b). Wild-type and zrt3 mutant cells bearing a ZRE–lacZ reporter were grown in a zinc-limiting medium (LZM) supplemented over a range of zinc concentrations and then assayed for β-galactosidase activity. Despite the higher accumulation of zinc in the zrt3 mutant (Figure 4A), more extracellular zinc was required to repress the ZRE–lacZ expression in this strain than in wild-type cells (Figure 5A). Interestingly, the increased reporter expression occurred over the same range of zinc concentrations (i.e. >10 µM) that resulted in greater zinc accumulation in the zrt3 mutant. The observed increase in ZRE–lacZ gene expression in the zrt3 mutant was also reflected in increased zinc uptake activity measured in these strains (data not shown), indicating that the effect is not an artifact of the lacZ reporter. These results showed that the excess zinc accumulated by zrt3 mutants is not detectable by the zinc-sensing mechanism of the Zap1p transcription factor. Thus, the zinc may be compartmentalized within an organelle.
Fig. 5. Effects of the zrt3 mutation on zinc-responsive gene expression. (A) Wild-type (DY1457) and zrt3 (CMK1) strains transformed with the pDg2 ZRE–lacZ reporter fusion were grown in LZM supplemented with the indicated concentration of ZnCl2 and assayed for β-galactosidase activity. (B) The same strains were grown in SD medium supplemented with 0 or 10 µM ZnCl2 and assayed for β-galactosidase activity. For (A) and (B), data points represent the means of three replicates from a representative experiment and the error bars indicate ± 1 SD.
An even more striking result was observed when zrt3 mutant cells were grown in a standard minimal medium, SD. In SD, the zrt3 mutant accumulated approximately the same level of zinc as wild type (data not shown), yet ZRE–lacZ activity was >25-fold higher in the zrt3 mutant (Figure 5B). This effect could be suppressed by adding 10 µM Zn2+ to the medium, indicating that in the unsupplemented medium the induction resulted from an apparent cytoplasmic/nuclear zinc deficiency. It is not yet known why the effect of the zrt3 mutation is more pronounced in SD versus LZM medium.
Zrt3p is a vacuolar membrane protein
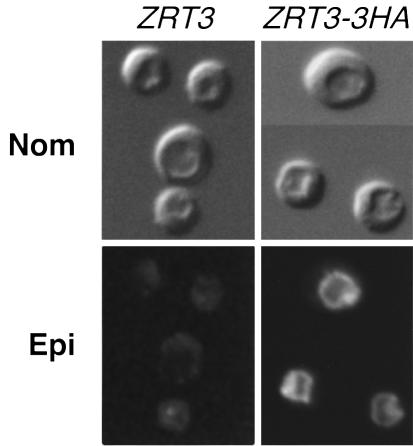
One hypothesis that explains these data is that Zrt3p is a zinc transporter responsible for releasing the metal from an intracellular organelle. If zinc transport into this compartment was mediated by other transporters, loss of Zrt3p activity would result in the trapping of zinc within the compartment where it would be unavailable both for controlling Zap1p and for other zinc-dependent processes. To identify the subcellular location of Zrt3p, the protein was epitope tagged with three repeats of the hemagglutinin (HA) epitope. A single copy of the tagged gene was expressed in a zrt3 mutant strain from its own promoter. The zinc accumulation phenotype of the zrt3 mutant was fully complemented by this plasmid (data not shown), indicating that the Zrt3p-3HA fusion was functional. Strains expressing the epitope-tagged and wild-type ZRT3 alleles were grown in zinc-limiting conditions to induce ZRT3 expression, and the distribution of the protein in the cell was determined by indirect immunofluorescence microscopy (Figure 6). While no fluorescence was observed in cells expressing the untagged allele, Zrt3p-3HA fluorescence was seen to outline the periphery of the vacuole, which was clearly visible by Nomarski optics as a single large organelle. These observations demonstrate that Zrt3p is located in the vacuole membrane, and implicate this compartment as the site of zinc accumulation in the zrt3 mutant.
Fig. 6. Zrt3p is localized to the vacuolar membrane. A zrt3 mutant (CMK1) expressing an epitope-tagged version of Zrt3p (ZRT3-3HA) or the untagged allele (pCM2, ZRT3) were grown to exponential phase in LZM with 1 µM ZnCl2. The cells were fixed, probed for the HA epitope, and viewed by Nomarski or epifluorescence. The indentations visible by Nomarski optics correspond to the vacuoles.
An intracellular zinc store buffers against changes in zinc availability
Previous studies supported a role for the vacuole in zinc detoxification, but it was not tested whether this sequestered zinc was available for subsequent use in zinc-deficient conditions. Our studies of ZRT3 suggested that the release of vacuolar zinc might help buffer changes in the labile zinc pool. To investigate this question, wild-type cells containing a ZRE–lacZ reporter gene were grown in a zinc-replete medium (SD) supplemented with a range of zinc concentrations. Cell-associated zinc levels were observed to increase in tandem with extracellular zinc concentrations (Figure 7A). Aliquots of these same cells were washed free of extracellular zinc and inoculated into a zinc-deficient medium (LZM with no added zinc). At time 0, all four samples showed similar low levels of ZRE–lacZ expression, indicating that the cells were zinc replete (Figure 7B). After 4 h of growth, a very different result was obtained: the cells grown initially in the unsupplemented medium induced ZRE–lacZ expression to a high level, while cells grown in higher zinc concentrations induced expression to a lesser degree.
Fig. 7. The mobilization of an intracellular zinc store is dependent on Zrt3p activity. (A) Wild-type (DY1457) cells transformed with a ZRE–lacZ fusion (pDg2) were grown in SD medium supplemented with the indicated amount of added ZnCl2. Cells were harvested, washed with EDTA, and cell-associated zinc levels determined by AAS. Data are the means of three independent experiments (error bars indicate ± 1 SD). (B) The same cells as in (A) were inoculated into a zinc-deficient medium (LZM with no added zinc) and assayed for β-galactosidase activity after 0 and 4 h. (C) Wild-type (DY1457) and zrt3 mutant (CMK1) strains bearing pDg2 were grown to exponential phase in a high zinc medium (SD plus 1 mM ZnCl2), washed free of extracellular zinc, and reinoculated into LZM without added ZnCl2. Cells were harvested at the indicated times and assayed for β-galactosidase activity. The inset graph shows the β-galactosidase activity over the full time course. For (B) and (C), data points represent the means of three replicates from a representative experiment and the error bars indicate ± 1 SD.
These results are consistent with an intracellular zinc store being released during the transition from zinc-replete to zinc-deficient conditions. To test whether Zrt3p was required for this mobilization, we compared ZRE–lacZ reporter gene induction in wild-type and zrt3 strains grown initially in high zinc conditions (SD + 1 mM zinc) and then inoculated into zinc-deficient media. Both strains were initially zinc replete and had low levels of ZRE–lacZ expression (Figure 7C). Upon transfer to zinc-deficient conditions, wild-type cells showed little induction of the reporter during the first 6 h of growth and maximum expression was attained after 20 h. In contrast, detectable induction of the ZRE–lacZ occurred in the zrt3 mutant after only 2 h and maximum induction occurred after only 10–12 h in zinc-deficient conditions. These observations indicate that Zrt3p is required to mobilize zinc stores during the transition from zinc-replete to zinc-deficient conditions.
Mutations in cot1 and zrc1 are epistatic to zrt3
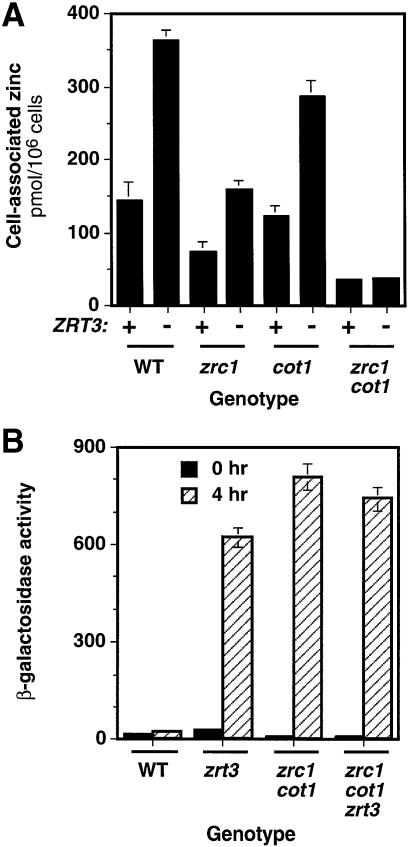
Previous studies showed that Zrc1p and Cot1p are vacuolar proteins and suggested that they may function to detoxify zinc (Kamizono et al., 1989; Conklin et al., 1992, 1994; Li and Kaplan, 1998). These observations, along with their membership in the CDF transporter family, indicated that Zrc1p and Cot1p are responsible for transporting zinc into the vacuole. If this hypothesis and our proposed role of Zrt3p are correct, then zrc1 and cot1 mutations should reduce zinc transport into the vacuole and hence limit the overaccumulation of zinc caused by the zrt3 mutation. To test this prediction, we generated a set of isogenic strains carrying all possible combinations of the zrt3, zrc1 and cot1 mutations. Cell-associated zinc levels were measured in these strains after growth in LZM supplemented with 1 mM zinc (Figure 8A); this medium was used instead of SD because zrc1 cot1 double mutants are hypersensitive to zinc in the absence of chelators. The zrc1 and cot1 single mutations both reduced zinc accumulation, with the zrc1 mutation having the greater effect. Inactivation of both genes reduced zinc accumulation even further. This observation supports the hypothesis that these two proteins overlap in function. As expected, deletion of ZRT3 increased zinc accumulation in ZRC1 COT1 cells. Zinc accumulation also increased when the zrt3 mutation was combined with either the zrc1 or cot1 mutations. However, the zrt3 mutation had no effect on zinc accumulation in a zrc1 cot1 double mutant. These results are consistent with Zrc1p and Cot1p together transporting zinc into the same compartment, presumably the vacuole, from which Zrt3p releases it.
Fig. 8. Effects of zrc1 and cot1 mutations on zinc accumulation and the generation of intracellular zinc stores. (A) Strains of the indicated genotypes (CM100–107) were grown to exponential phase in LZM supplemented with 1 mM ZnCl2, harvested, washed, and assayed for cell-associated zinc levels by AAS. Data are the means of three independent experiments and the error bars indicate ± 1 SD. (B) Cells of the indicated genotypes bearing a ZRE–lacZ reporter (pDg2-1-LEU2) were grown in LZM supplemented with 1 mM ZnCl2, harvested, and inoculated into LZM with no added zinc. After 0 and 4 h incubation, the cells were harvested and assayed for β-galactosidase activity. Data points represent the means of three replicates from a representative experiment and the error bars indicate ± 1 SD.
This model of vacuolar zinc storage also suggested that zrc1 cot1 mutants would be unable to generate a zinc store in replete conditions to use later in deficient conditions. To test this prediction, we assessed the induction of ZRE–lacZ expression in zinc-replete wild type, zrt3, zrc1 cot1 and zrc1 cot1 zrt3 mutant strains subsequently exposed to a zinc-limiting medium. The experimental protocol used was identical to that described in Figure 7B except that cultures were initially grown in LZM + 1 mM zinc to prevent toxicity to the zrc1 cot1 mutants. Activity of the ZRE–lacZ reporter was initially low in all strains, indicating their zinc-replete status (Figure 8B). After 4 h in zinc-limiting medium, wild-type cells showed little ZRE–lacZ induction, while each mutant strain had highly induced expression. These results indicate that the inability to generate a zinc store in replete media prevented these strains from buffering the cytoplasmic pool against decreases in the external zinc concentration. Interestingly, after 4 h of zinc-deficient growth, ZRE–lacZ expression in the zrt3 strain was almost as high as in the zrc1 cot1 strain. This similar induction occurred despite an ∼8-fold difference in the initial zinc content of these strains (Figure 8A), suggesting that the loss of Zrt3p activity effectively prevented the release of vacuolar zinc. As expected, the zrc1 cot1 zrt3 triple mutant had no greater effect on ZRE–lacZ induction than the zrc1 cot1 combination.
Discussion
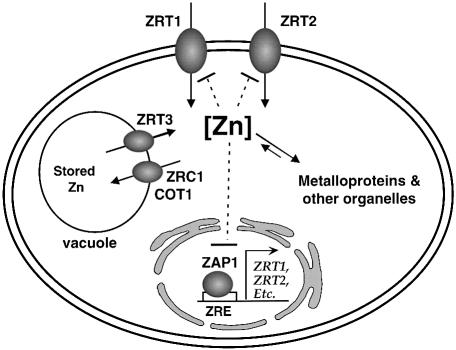
Our studies of Zrt3p have led to new insights into processes of zinc homeostasis in S.cerevisiae (Figure 9). We propose that the zinc transported across the plasma membrane by Zrt1p and Zrt2p enters a labile pool of zinc in the cytoplasm. This pool supplies zinc to organelles such as the mitochondria and endoplasmic reticulum and to zinc metalloproteins synthesized in the cytoplasm. In replete cells, excess zinc is transported into the vacuole via Zrc1p and Cot1p. Our model also proposes that vacuolar zinc is stored in a usable form. During the transition from zinc-replete to zinc-deficient growth conditions, the labile pool of cytoplasmic zinc decreases, allowing for increased activity of the Zap1p transcription factor. Zap1p, in turn, increases expression of the ZRT3 gene and the resulting rise in Zrt3p activity promotes zinc transport out of the vacuole into the cytoplasm, where it becomes available for use.
Fig. 9. Zinc homeostasis in yeast. Zinc enters the cell via the Zrt1p and Zrt2p transporters. Once in the cytoplasm, the metal is bound by metalloproteins or transported into organelles such as the mitochondria. In zinc-replete cells, Zrc1p and Cot1p transport zinc into the vacuole, where it is stored in a less toxic form. The Zap1p transcription factor is repressed by an intracellular pool of labile zinc. When zinc becomes limiting, Zap1p activity increases transcription of the ZRT1, ZRT2 and ZRT3 genes. Increased expression of the Zrt3p transporter mobilizes zinc stores from the vacuole into the cytoplasm to replenish the labile zinc pool. Paradoxically, the ZRC1 gene is also induced by Zap1p, suggesting a need to maintain zinc flux through the vacuole in zinc-limited cells. If cellular zinc rises to extremely high levels, e.g. when zinc-deficient cells are exposed to high zinc, intracellular zinc signals the endocytosis of the Zrt1p and Zrt2p transporters (Gitan et al., 1998). These proteins are subsequently degraded in the vacuole. Thus, zinc homeostasis represents a balance between these transcriptional and post-translational regulatory systems and the transporter proteins they control.
A number of observations support this hypothesis of vacuolar zinc storage. Zinc accumulation within the vacuoles of zinc-replete cells was first observed by dithiozone staining (Bilinski and Miller, 1983). White and Gadd (1987) demonstrated that increasing extracellular zinc levels resulted in higher cell-associated zinc, and that the majority of this zinc co-purified with the vacuole during subcellular fractionation. Furthermore, mutants defective for vacuolar biogenesis or acidification are zinc sensitive, suggesting that accumulation in the vacuole is needed to detoxify the metal efficiently (Eide et al., 1993; Ramsay and Gadd, 1997).
Zrc1p and Cot1p appear to be the transporters responsible for vacuole zinc accumulation. They are both vacuolar membrane proteins (Li and Kaplan, 1998) and are members of the CDF family of transporters (Paulsen and Saier, 1997). Other members, e.g. ZnT-1, ZnT-2, etc., serve as zinc transporters in mammals and plants. Overexpression of ZRC1 or COT1 increased zinc tolerance, suggesting an increased capacity to sequester zinc in the vacuole. Mutation of ZRC1 and COT1 decreased zinc tolerance, indicating a loss of this ability. Furthermore, we showed that cellular zinc levels are also reduced in zrc1 and cot1 mutants. Genetic evidence argues that Zrc1p and Cot1p at least partially overlap in function; zinc tolerance (our unpublished results) and zinc accumulation are reduced to a greater extent in the zrc1 cot1 double mutant than in either single mutant strain. The zinc sensitivity of vacuolar acidification mutants further suggests that the low pH of the vacuolar lumen may provide the driving force for these transporters. A Zn2+/H+ antiport mechanism has been detected in purified yeast vacuolar vesicles and may represent the activity of Zrc1p and/or Cot1p (Okorokov et al., 1985; White and Gadd, 1987).
Our results also indicate that the vacuole provides an accessible pool of zinc for use during the transition from replete to deficient conditions. The accumulation of a high level of cell-associated zinc during growth in zinc-replete medium correlated with the reduced induction of a ZRE–lacZ fusion following transfer to zinc-deficient medium. The observed effect of zrc1 and cot1 mutations on this induction clearly links this zinc storage pool and the vacuole. Cells that are unable to generate a zinc store under replete conditions induce Zap1p-dependent expression more rapidly after transfer to low zinc conditions. Zrt3p is apparently the transporter responsible for mobilizing vacuolar zinc as cells make the transition from replete to deficient conditions. First, Zrt3p, like Zrc1p and Cot1p, is found in the vacuole membrane. Secondly, ZRT3 expression is induced in zinc-limited cells by Zap1p. Therefore, Zrt3p is present in the right place and at the right time to mobilize stored zinc. A third factor underlying our hypothesis is the membership of Zrt3p in the ZIP family of transporters. Functional data obtained with several of these proteins suggest a conserved role for this family in the transport of metal ions, especially zinc. Consistent with the sequence similarity of Zrt3p with Zrt1p and Zrt2p, genetic evidence indicated that these three proteins have overlapping functions. This result indicated that all three proteins contribute to the labile zinc pool required for cell growth. It was, therefore, remarkable that mutation and overexpression of ZRT3 had opposite effects to similar alterations in expression of the plasma membrane transporters (Zhao and Eide, 1996a,b). Thus, while Zrt3p plays an analogous role to Zrt1p and Zrt2p by supplying zinc to the cytoplasm, it performs this function in the vacuole rather than in the plasma membrane. Finally, using the ZRE–lacZ reporter as a bioassay of the labile pool of zinc, we demonstrated that despite having excess zinc, the zrt3 mutant is unable to mobilize that store for growth and must rely on up-regulation of plasma membrane transporters. These data indicate that regulation of Zrt3p activity plays the major role in controlling the mobilization of vacuolar zinc stores.
Our model raises a number of intriguing questions about yeast zinc homeostasis. First, why do zrt3 mutants not show a growth defect in low zinc media when our model predicts that they cannot mobilize intracellular zinc stores? The answer to this question is apparent in Figure 7C. The zrt3 mutants compensate for their inability to mobilize zinc stores by up-regulating zinc uptake activity earlier in the transition from replete to deficient conditions. The growth defect of a zrt1 zrt2 zrt3 triple mutant in low zinc is consistent with this hypothesis; the inability of this strain to increase zinc uptake would limit its ability to replenish diminishing zinc stores.
A second question raised is why does accumulation occur when expression of ZRT3 is low and not when it is expressed at higher levels? One explanation for this observation is that, in low zinc concentrations, Zrc1p and Cot1p are unable to compete for their substrate with zinc-binding proteins and other organellar transporters. Because the resulting vacuolar zinc levels are low, there is little impact from the loss of Zrt3p activity. As extracellular zinc levels rise, the cytoplasmic labile pool also increases, ultimately saturating high affinity zinc-binding sites; excess zinc is then available for Zrc1p- and Cot1p-mediated transport. It is under these conditions that a lack of Zrt3p activity can be observed. This hypothesis is consistent with our observation that the phenotypic effects of zrt3 mutations are only apparent in increasing zinc concentrations after maximal growth is attained (∼10 µM in LZM) and the presumed high affinity sites are likely to be fully occupied. Based on the measured cell-associated zinc levels, we estimate that the minimum zinc content required for optimal growth is ∼1.5 × 107 atoms/cell. Zinc levels lower than this value sustain only poor growth, while higher levels apparently trigger vacuolar storage.
The observation that zinc accumulates to a higher level in zinc-replete zrt3 mutants when compared with wild-type cells indicates that zinc cycling through the vacuole occurs under these conditions. Some vacuolar zinc cycling may also occur in zinc-deficient cells. This is suggested by our recent observation that ZRC1 is also a Zap1p target gene (T.Lyons, A.Gasch, L.A.Gaither, D.Botstein, P.Brown and D.Eide, unpublished observation); ZRC1 is induced by ∼2-fold in zinc-limited cells. Why would zinc-limited cells increase expression of both vacuolar influx and efflux transporters? This regulation suggests that it may be important to maintain a zinc flux through the vacuole even in zinc-deficient cells. While the function of this zinc cycling is not clear, it may be needed to maintain a sufficient supply of zinc to preserve the function of zinc-dependent vacuolar enzymes such as metalloproteases.
Another significant aspect of our studies of Zrt3p is that its identification adds many new members to the ZIP family of metal ion transporters. The known ZIP family members were previously limited to eukaryotic organisms only. This observation led to the suggestion that the ZIP family arose after divergence of eukaryotic and prokaryotic kingdoms (Eng et al., 1998). However, with the identification of Zrt3p, the family can now be expanded to include members from both archaea and eubacteria. This suggests that the ZIP family is much more ancient in origin than was previously supposed. Given that all of the members of the family that have been examined experimentally, including Zrt3p, are involved in metal ion transport, we suggest that the prokaryotic members may play similar roles in metal ion accumulation. This is an exciting prospect given how little is known about metal ion transporters in these organisms.
The characterization of Zrt3p also suggests that some ZIP transporters may be involved in mobilizing stored zinc from intracellular compartments in other eukaryotes. Cytoplasmic zinc-binding proteins called metallothioneins (MTs) have been proposed to be a potential source of stored zinc (Hamer, 1986). However, studies of MT knockout mice indicate that these proteins play a minor role in zinc storage and demonstrate that some other storage site must exist (Palmiter, 1998). Clues to the identity of this site come from several studies using zinc-activated fluorophores (e.g. zinquin) to observe the distribution of labile intracellular zinc. These studies showed that labile zinc is detectable in membrane-bound vesicles in a variety of mammalian cells (Zalewski et al., 1993; Palmiter et al., 1996a). The fluorescence intensity of these vesicles increased in zinc-treated cells, indicating that zinc accumulates in these compartments in response to higher zinc availability. Intriguingly the zinquin-staining compartment of the cell co-localized with the ZnT-2 zinc transporter, a CDF protein related to Zrc1p and Cot1p, which has recently been localized to late endosomes (Kobayashi et al., 1999). Taken together, these data suggest that the mammalian late endosome is analogous to the yeast vacuole as a zinc storage compartment. Thus, ZIP transporters may mediate both zinc uptake and mobilization of intracellular zinc stores in all eukaryotes.
Materials and methods
Sequence analysis
Searching of the yeast genome sequence for ZRE-containing promoters used PATMATCH (http://genome-www2.stanford.edu/cgi-bin/SGD/PATMATCH), computer database comparisons were performed using BLAST (Altschul et al., 1997), potential transmembrane domains were identified with TOP-PRED II (Claros and Von Heijne, 1994) and multiple sequence alignments were generated with CLUSTALX (Thompson et al., 1997).
Yeast strains and growth conditions
Saccharomyces cerevisiae strains used are described in Table I. Yeast was grown in YPD or in SD medium with 2% glucose and necessary auxotrophic requirements. Where indicated, SD was made zinc limiting by adding 1 mM EDTA and 50 µM ZnCl2. The added zinc is required to provide sufficient free metal ion for growth. LZM was prepared as described previously (Gitan et al., 1998). Cell number per milliliter of yeast suspension was determined by measuring the optical density at 600 nm (A600) and comparing with a standard curve. The CMK1 zrt3 deletion mutant was generated using a PCR-mediated gene disruption method (Wach, 1996). The oligonucleotides used were (5′ ZRT3) 5′-CCAGCGAATCGTTGGTACACTGTGCTGATACGGTGACCACGTACGCTGCAGGTCGC-3′ and (3′ ZRT3) 5′-GCACCAATTTTCAAGCAAGTTTGCCTGAGCTATGGGACTGTATCGATGAATTCGAGCTCG-3′. These primers were used to amplify the kanMX4 module (Wach, 1996), and the resulting PCR product, which contains the Kanr gene flanked by 40 bp of the ZRT3 5′ and 3′ untranslated regions, was used to transform DY1457. G418-resistant colonies were selected and correct deletion of ZRT3 was verified by PCR. The CM100–CM107 strains were derived from crosses of CMK1 with the Δzrc1 (zrc1::HIS3) and Δcot1 (cot1::URA3) strains (a gift from Jerry Kaplan), which are isogenic to DY1457. Strains CM30, 34 and 37 were generated by crossing CMK1 with ZHY3 (Zhao and Eide, 1996b).
Table I. Yeast strains used.
| Strain | Relevant genotype | Full genotype | Source |
|---|---|---|---|
| DY1457 | wild type | MATα ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 | Zhao and Eide (1997) |
| ZHY6 | zap1Δ | MATα ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zap1::TRP1 | Zhao and Eide (1997) |
| ZHY7 | ZAP1-1up | MATα ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 ZAP1-1up | Zhao and Eide (1997) |
| CMK1 | zrt3Δ | MATα ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrt3::KANR | this work |
| CM30 | wild type | MATα ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 | this work |
| CM34 | zrt1Δ zrt2Δ | MATα ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrt1::LEU2 zrt2::HIS3 | this work |
| CM37 | zrt1Δ zrt2Δ zrt3Δ | MATa ade6 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrt1::LEU2 zrt2::HIS3 zrt3::KANR | this work |
| CM100 | wild type | MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 | this work |
| CM101 | zrt3Δ | MATa can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrt3::KANR | this work |
| CM102 | zrc1Δ | MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrc1::HIS3 | this work |
| CM103 | cot1Δ | MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 cot1::URA3 | this work |
| CM104 | cot1Δ zrc1Δ | MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrc1::HIS3 cot1::URA3 | this work |
| CM105 | zrc1Δ zrt3Δ | MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrc1::HIS3 zrt3::KANR | this work |
| CM106 | cot1Δ zrt3Δ | MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 cot1::URA3 zrt3::KANR | this work |
| CM107 | cot1Δ zrc1Δ zrt3Δ | MATa can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrc1::HIS3 cot1::URA3 zrt3::KANR | this work |
DNA manipulations
The ZRE–lacZ reporters used in this work were pGI3 (Zhao and Eide, 1996a), pDg2 (Zhao et al., 1998) and pDG2-1-LEU2 (a pDg2 derivative constructed by A.Bird). The pAG3 ZRT3 promoter–lacZ fusion was constructed by amplifying the ZRT3 promoter (–1000 to the ATG start codon) from DY1457 genomic DNA, using the primers AG1 (5′-CGAATTCGATAGCATTTCCGAAGGC-3′) and AG2 (5′-CGGGATCCATTTTCTTGCGTTTCTTCAC-3′). The resulting 1 kb fragment was cloned into YEp353 (Myers et al., 1986) using the BamHI and EcoRI sites in the primers. The ZRT3 open reading frame and flanking 1000 bp of promoter region were amplified from DY1457 genomic DNA using the primers AG2 and CM1 (5′-CGAATTCGATAGCATTTCCGAAGGC-3′). The resulting 2.7 kb fragment was cloned into the BamHI and EcoRI sites of the yeast shuttle vector pFL39 (Bonneaud et al., 1991) to generate pCM2. An epitope-tagged ZRT3 allele was generated using inverse PCR (Gama and Breitwieser, 1999) with primers designed to add three copies of the Haemophilus influenzae HA epitope to the C-terminus of the Zrt3p in pCM2. The primers used were: CM3-5′-GTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTAAGTGAAAAGGGCACTCG-3′ and 5′-GTCCCGGACTATGCAGGATATCCA TATGACGTTCCAGATTACGCTTGATAAATTAAAACTA-3′. The ZRT3-3HA fusion was verified by sequencing and ZRT3 function was confirmed by complementing the zinc-accumulation phenotype of a zrt3 mutant strain.
β-galactosidase assays and atomic absorption spectroscopy (AAS)
β-galactosidase activity was measured by the method of Guarente (1983). Cells were harvested in exponential growth phase (A600 of 0.5–1.5) and activity was calculated as follows: (ΔA420 × 1000)/(min × ml of culture used × culture A600). Measurement of total cell-associated zinc was performed by AAS (Gitan et al., 1998).
Indirect immunofluorescence microscopy
Yeast cultures (50 ml) were grown in LZM supplemented with 1 µM ZnCl2 and harvested during exponential growth. The cells were resuspended in 0.1 M Tris–SO4 pH 9.3, 10 mM dithiothreitol and incubated for 10 min at 30°C. The cells were collected by centrifugation (5 min, 1000 g), washed once in phosphate-buffered saline (PBS), resuspended in cold methanol and fixed for 20 h at –20°C. Fixed cells were washed three times in solution A (40 mM KPO4 pH 6.5, 0.5 mM MgCl2, 1.2 M sorbitol) and resuspended in solution A containing 10 mM β-mercaptoethanol and lyticase enzyme (Boehringer Mannheim; 2 U/A600 unit of cells). The cells were digested for 2 h at 30°C, and the resulting spheroplasts were washed twice with PBS and bound to polylysine-coated microscope slides. The cells were incubated with PBS containing 1 mg/ml bovine serum albumin (BSA) for 1 h at 37°C. A 1:500 dilution of the primary mouse monoclonal anti-HA antibody (12CA5; BABCO) in PBS/BSA was applied to the slides and incubated for 1 h at 37°C. The slides were then washed with 20 ml of PBS, and a 1:500 dilution of goat anti-mouse IgG antibody conjugated to Alexa 488 (Molecular Probes) was added and incubated for 1 h at 37°C. After five washes with PBS, the cells were mounted in 50% glycerol and viewed with epifluorescence and Nomarski optics.
Acknowledgments
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01 GM56285).
References
- Altschul S.F., Madden,T.L., Schaffer,A.A., Zhang,J., Zhang,Z., Miller, W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski C.A. and Miller,J.J. (1983) Translocation of zinc from vacuole to nucleus during yeast meiosis. Can. J. Genet. Cytol., 25, 415–419. [DOI] [PubMed] [Google Scholar]
- Bode H.-P., Dumschat,M., Garotti,S. and Fuhrmann,G.F. (1995) Iron sequestration by the yeast vacuole. Eur. J. Biochem., 228, 337–342. [PubMed] [Google Scholar]
- Bonneaud N., Ozier-Kalogeropoulos,O., Li,G.Y., Labouesse,M., Minvielle-Sebastia,L. and Lacroute,F. (1991) A family of low and high copy replicative, integrative and single-stranded S.cerevisiae/E.coli shuttle vectors. Yeast, 7, 609–615. [DOI] [PubMed] [Google Scholar]
- Claros M.G. and Von Heijne,G. (1994) TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci., 10, 685–686. [DOI] [PubMed] [Google Scholar]
- Conklin D.S., McMaster,J.A., Culbertson,M.R. and Kung,C. (1992) COT1, a gene involved in cobalt accumulation in Saccharomyces cerevisiae. Mol. Cell. Biol., 12, 3678–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D.S., Culbertson,M.R. and Kung,C. (1994) Interactions between gene products involved in divalent cation transport in Saccharomyces cerevisiae. Mol. Gen. Genet., 244, 303–311. [DOI] [PubMed] [Google Scholar]
- Costello L.C., Liu,Y., Zou,J. and Franklin,R.B. (1999) Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J. Biol. Chem., 274, 17499–17504. [DOI] [PubMed] [Google Scholar]
- Dunn T., Gable,K. and Beeler,T. (1994) Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem., 269, 7273–7278. [PubMed] [Google Scholar]
- Eide D., Bridgham,J.T., Zhong,Z. and Mattoon,J. (1993) The vacuolar H+-ATPase of Saccharomyces cerevisiae is required for efficient copper detoxification, mitochondrial function, and iron metabolism. Mol. Gen. Genet., 241, 447–456. [DOI] [PubMed] [Google Scholar]
- Eng B.H., Guerinot,M.L., Eide,D. and Saier,M.H. (1998) Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J. Membr. Biol., 166, 1–7. [DOI] [PubMed] [Google Scholar]
- Gaither L.A. and Eide,D.J. (2000) Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem., 275, 5560–5564. [DOI] [PubMed] [Google Scholar]
- Gama L. and Breitwieser,G.E. (1999) Generation of epitope-tagged proteins by inverse PCR mutagenesis. Biotechniques, 26, 814–816. [DOI] [PubMed] [Google Scholar]
- Gitan R.S., Lou,H., Rodgers,J., Broderius,M. and Eide,D. (1998) Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem., 273, 28617–28624. [DOI] [PubMed] [Google Scholar]
- Grotz N., Fox,T., Connolly,E., Park,W., Guerinot,M.L. and Eide,D. (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl Acad. Sci. USA, 95, 7220–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. (1983) Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol., 101, 181–191. [DOI] [PubMed] [Google Scholar]
- Guerinot M.L. and Eide,D. (1999) Zeroing in on zinc uptake in yeast and plants. Curr. Opin. Plant Biol., 2, 244–249. [DOI] [PubMed] [Google Scholar]
- Hamer D.H. (1986) Metallothionein. Annu. Rev. Biochem., 55, 913–951. [DOI] [PubMed] [Google Scholar]
- Huang L. and Gitschier,J. (1997) A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nature Genet., 17, 292–297. [DOI] [PubMed] [Google Scholar]
- Kamizono A., Nishizawa,M., Teranishi,Y., Murata,K. and Kimura,A. (1989) Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae. Mol. Gen. Genet., 219, 161–167. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Beuchat,M.H., Lindsay,M., Frias,S., Palmiter,R.D., Sakuraba,H., Parton,R.G. and Gruenberg,J. (1999) Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nature Cell Biol., 1, 113–118. [DOI] [PubMed] [Google Scholar]
- Korshunova Y.O., Eide,D., Clark,W.G., Guerinot,M.L. and Pakrasi,H.B. (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol., 40, 37–44. [DOI] [PubMed] [Google Scholar]
- Li L. and Kaplan,J. (1998) Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity. J. Biol. Chem., 273, 22181–22187. [DOI] [PubMed] [Google Scholar]
- Murgia C., Vespignani,I., Cerase,J., Nobili,F. and Perozzi,G. (1999) Cloning, expression and vesicular localization of zinc transporter Dri/ZnT4 in intestinal tissue and cells. Am. J. Physiol., 277, G1231–G1239. [DOI] [PubMed] [Google Scholar]
- Myers A.M., Tzagoloff,A., Kinney,D.M. and Lusty,C.J. (1986) Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene, 45, 299–310. [DOI] [PubMed] [Google Scholar]
- Nies D.H. and Silver,S. (1995) Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol., 14, 186–199. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Igarashi,K. and Kakinuma,Y. (1998) Proton gradient-driven nickel uptake by vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Bacteriol., 180, 1962–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorokov L.A., Kulakovskaya,T.V., Lichko,L.P. and Polorotova,E.V. (1985) H+/ion antiport as the principal mechanism of transport systems in the vacuolar membrane of the yeast Saccharomyces carlsbergensis. FEBS Lett., 192, 303–306. [DOI] [PubMed] [Google Scholar]
- Paidhungat M. and Garrett,S. (1998) Cdc1 and the vacuole coordinately regulate Mn2+ homeostasis in the yeast Saccharomyces cerevisiae. Genetics, 148, 1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D. (1998) The elusive function of metallothioneins. Proc. Natl Acad. Sci. USA, 95, 8428–8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D. and Findley,S.D. (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J., 14, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D., Cole,T.B. and Findley,S.D. (1996a) ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J., 15, 1784–1791. [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D., Cole,T.B., Quaife,C.J. and Findley,S.D. (1996b) ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA, 93, 14934–14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen I.T. and Saier,M.H. (1997) A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol., 156, 99–103. [DOI] [PubMed] [Google Scholar]
- Ramsay L.M. and Gadd,G.M. (1997) Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol. Lett., 152, 293–298. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson,T.J., Plewniak,F., Jeanmougin,F. and Higgins,D.G. (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res., 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B.L. and Falchuk,K.H. (1993) The biochemical basis of zinc physiology. Physiol. Rev., 73, 79–118. [DOI] [PubMed] [Google Scholar]
- Wach A. (1996) PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast, 12, 259–265. [DOI] [PubMed] [Google Scholar]
- White C. and Gadd,G.M. (1987) The uptake and cellular distribution of zinc in Saccharomyces cerevisiae. J. Gen. Microbiol., 133, 727–737. [Google Scholar]
- Zalewski P.D., Forbes,I.J. and Betts,W.H. (1993) Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II). Biochem. J., 296, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. and Eide,D. (1996a) The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl Acad. Sci. USA, 93, 2454–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. and Eide,D. (1996b) The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem., 271, 23203–23210. [DOI] [PubMed] [Google Scholar]
- Zhao H. and Eide,D.J. (1997) Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Mol. Cell. Biol., 17, 5044–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Butler,E., Rodgers,J., Spizzo,T., Duesterhoeft,S. and Eide,D. (1998) Regulation of zinc homeostasis in yeast by binding of the ZAP1 transcriptional activator to zinc-responsive promoter elements. J. Biol. Chem., 273, 28713–28720. [DOI] [PubMed] [Google Scholar]