Abstract
The internalization step of endocytosis in yeast requires actin and sterols for maximum efficiency. In addition, many receptors and plasma membrane proteins must be phosphorylated and ubiquitylated prior to internalization. The Saccharomyces cerevisiae end8-1 mutant is allelic to lcb1, a mutant defective in the first step of sphingoid base synthesis. Upon arrest of sphingoid base synthesis a rapid block in endocytosis is seen. This block can be overcome by exogenous sphingoid base. Under conditions where endogenous sphingosine base synthesis was blocked and exogenous sphingoid bases could not be converted to phosphorylated sphingoid bases or to ceramide, sphingoid bases could still suppress the endocytic defect. Therefore, the required lipid is most likely a sphingoid base. Interestingly, sphingoid base synthesis is required for proper actin organization, but is not required for receptor phosphorylation. This is the first case of a physiological role for sphingoid base synthesis, other than as a precursor for ceramide or phosphorylated sphingoid base synthesis.
Keywords: actin/ceramide/endocytosis/lcb1/sphingoid base
Introduction
The process of membrane biogenesis requires the selective capture of membrane and soluble components into budding vesicles, which are then directed to the proper target compartment for fusion. This process must be highly regulated to account for the observed fidelity of intracellular transport. Several components have been implicated in the regulation of this fidelity including proteins that form vesicle coats (Cosson and Letourneur, 1997; Kirchhausen et al., 1997; Kuehn and Schekman, 1997), components required for fusion (Rothman, 1996; Novick and Zerial, 1997), and various lipids (Horvath et al., 1994; Alb et al., 1996; De Camilli et al., 1996; Harder and Simons, 1997; Schmidt et al., 1999; Thiele et al., 2000). While the functions defined for the different types of protein components seem to be similar for different vesicular trafficking steps, the roles of the various lipids are not yet well defined and may be different. Functions could include protein recruitment for phosphoinositides (Martin and Kowalchyk, 1997), signaling in the case of diacylglycerol (Kearns et al., 1997), or structural roles in the lipid bilayer (Munn et al., 1999; Schmidt et al., 1999).
In yeast cells, and perhaps in mammalian cells as well, actin is required for the internalization step of endocytosis (Kübler and Riezman, 1993; Lamaze et al., 1997), but its precise function is unknown. Genetic screens have identified a large number of mutants affecting actin dynamics that have been shown to affect the internalization step of endocytosis (Raths et al., 1993; Munn and Riezman, 1994; Munn et al., 1995; Wendland et al., 1996). It is likely that actin acts directly in the process because only a specific subset of proteins that regulate the actin cytoskeleton are required (Geli and Riezman, 1998; Wendland et al., 1998). The α-factor receptor, as well as other plasma membrane proteins, are phosphorylated and ubiquitylated prior to their internalization and these events are essential for the process (Galan et al., 1996; Hicke and Riezman, 1996; Hicke et al., 1998; Hicke, 1999). Certain lipids, such as phosphoinositides and sterols, have been implicated in control of the internalization step of endocytosis in both yeast and mammalian cells (Haffner et al., 1997; Rapoport et al., 1997; Singer-Krüger et al., 1998; Munn et al., 1999; Rodal et al., 1999). Phosphorylated phosphoinositides have also been implicated in control of the actin cytoskeleton in both systems (Goldschmidt-Clermont et al., 1990; Sakisaka et al., 1997; Desrivières et al., 1998; He et al., 1998; Homma et al., 1998), although the precise function of these molecules remains to be discovered (Schmidt and Hall, 1998). Sphingoid bases have also been shown to play a role in regulation of amino acid permeases (Skrzypek et al., 1998).
Here, we show that synthesis of dihydrosphingosine (DHS) or phytosphingosine (PHS) is required for the internalization step of endocytosis in yeast. This defect can be overcome by adding back exogenous sphingoid bases. Using conditions where the sphingoid bases could not be converted to phosphorylated sphingoid bases or ceramide we could still suppress the endocytic defect. In the absence of sphingoid base the actin cytoskeleton was also not assembled correctly. These results suggest that sphingoid base plays an important role in the internalization step of endocytosis.
Results
The yeast end8-1 mutant has a temperature-sensitive defect in the internalization step of endocytosis witnessed by a defect in accumulation of the fluid-phase marker, lucifer yellow CH (LY), in the vacuole and a defect in internalization of the yeast pheromone, α-factor (Munn and Riezman, 1994). We cloned END8 and demonstrated that it is identical to LCB1, a gene required for expression of serine palmitoyltransferase activity, the first step in ceramide synthesis (Buede et al., 1991). Therefore, we refer to the end8-1 mutant as lcb1-100. Since ceramide can be converted into sphingolipids (Figure 1), we measured sphingolipid synthesis as an indirect measure of ceramide synthesis. Wild-type or mutant cells were grown at 24°C, then metabolically labeled with [3H]myoinositol at 24 and 37°C. Lipids were extracted, treated with mild base to identify sphingolipids, which are base-resistant, and separated by thin layer chromatography (TLC) (Figure 2A). Both wild-type and mutant cells were capable of synthesis of sphingolipids [inositolphosphorylceramide (IPC), mannosylated inositolphosphorylceramide (MIPC), mannosylated di-inositolphosphorylceramide (M(IP)2C)] at 24°C, although the lcb1-100 strain showed only 38% the level of synthesis found in the wild type at the same temperature. At 37°C, wild-type cells showed a 55% increase in synthesis of sphingolipids compared with at 24°C. An increase in ceramide synthesis upon shift to 37°C has been seen previously (Jenkins et al., 1997; Wells et al., 1998). In contrast, the lcb1-100 mutant showed a reduction in sphingolipid synthesis at 37°C, amounting to a 25-fold decrease in sphingolipid synthesis at 37°C when compared with the wild-type strain at the same temperature. This suggests that the lcb1-100 strain has temperature-sensitive serine palmitoyltransferase activity. The synthesis of M(IP)2C is much less affected than the other sphingolipids. This is most likely the consequence of a reduced flux through the pathway because it was also seen (Horvath et al., 1994) when yeast cells were treated with myriocin, an inhibitor of serine palmitoyltransferase. Since the ceramide synthesis pathway is partially functional at 24°C, it is interesting that the temperature-sensitive defect in endocytosis is very rapidly installed (<15 min; Munn and Riezman, 1994) (Figure 2B). Therefore, the endocytic defect is most likely to result from the rapid depletion or lack of an increase of an unstable compound. During the time period of the endocytosis assay, there is no significant turnover of total sphingolipids (our unpublished data; Dickson and Lester, 1999).
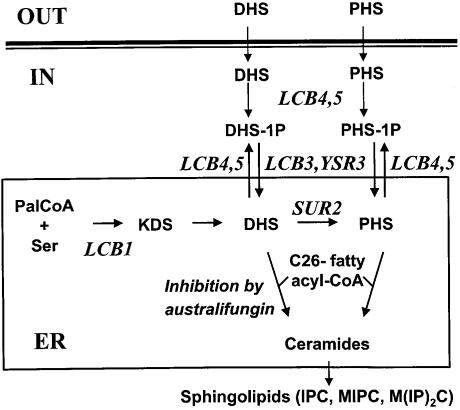
Fig. 1. Synthesis of sphingolipids in yeast from endogenous and exogenous precursors. DHS and PHS can be internalized by cells and phosphorylated by the LCB4 and LCB5 gene products to give DHS-1P and PHS-1P. The phosphorylated sphingoid bases can be dephosphorylated by the LCB3 and YSR3 gene products. LCB1 encodes a subunit of serine palmitoyltransferase. The SUR2 gene product converts DHS into PHS. Synthesis of ceramides from DHS or PHS and C26 fatty acyl-CoA is inhibited by australifungin. Ceramides are used to synthesize the sphingolipids IPC (inositolphosphorylceramide), MIPC (mannosylated inositolphosphorylceramide) and M(IP)2C (mannosylated di-inositolphosphorylceramide).
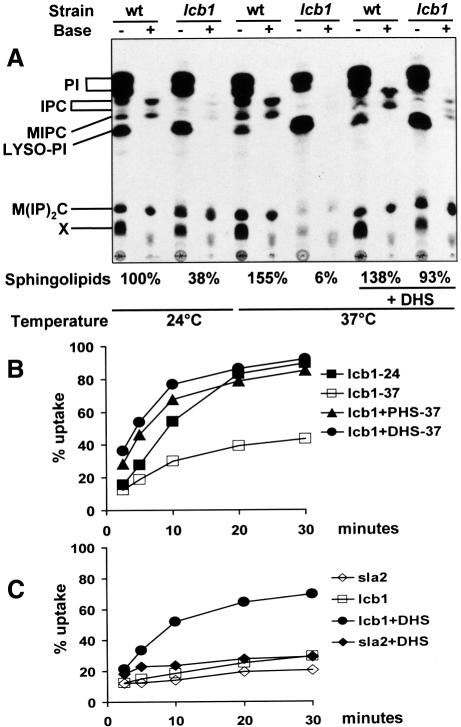
Fig. 2. Conditional sphingolipid synthesis and endocytosis in lcb1-100 cells. (A) Wild-type (RH1800, wt) or lcb1 mutant (RH3809) cells were grown overnight to A600 = 0.4, preshifted to the appropriate temperature for 15 min in the absence or presence of DHS, and labeled with [3H]myoinositol at 24 or 37°C in the absence or presence of DHS. Incorporation of [3H]myoinositol into the total lipid fraction was quantified and equal c.p.m. were applied to TLC plates, or treated with mild base to identify sphingolipids and applied to TLC plates. Incorporation of radioactivity into sphingolipids was quantified using a phosphorimager [sum of IPC + MIPC + M(IP)2C] and the relative amount of sphingolipid synthesis determined after setting the amount in wild-type cells at 24°C to 100%. PI, phosphatidylinositol; IPC; MIPC; LYSO-PI, lysophosphatidylinositol; M(IP)2C; X, unidentified lipid. The products were analyzed by TLC using solvent system I. (B) Yeast cells were grown overnight in SDYE. Internalization of [35S]α-factor by RH3809 cells was measured at 24 or at 37°C in SDYE in the presence or absence of DHS or PHS. (C) Yeast were grown overnight in YPUAD. Internalization of [35S]α-factor by RH3809 and RH1597 cells was measured in SDYE at 37°C in the presence or absence of DHS.
If an unstable intermediate in the pathway is required for endocytosis then adding back a sphingoid base to the lcb1-100 mutant at 37°C should restore endocytosis to the mutant cells. As expected, DHS or PHS restored endocytosis to lcb1-100 mutant cells (Figure 2B). Addition of DHS to the lcb1-100 mutant also restored synthesis of sphingolipids at 37°C (Figure 2A). PHS also restored sphingolipid synthesis under identical conditions (our unpublished data). To show that the suppression by DHS is due to the replenishment of sphingoid base and not a general suppression of endocytic defects, we added DHS to another mutant defective for the internalization step of endocytosis. The sla2-41 (end4) mutant (Raths et al., 1993) has a defective actin cytoskeleton (Holtzman et al., 1993) and was not suppressed by addition of DHS (Figure 2C). These results suggest that DHS and PHS specifically suppress the endocytic defect of the lcb1-100 mutant by restoring sphingoid bases. The difference seen between the internalization rates of the lcb1-100 mutant in Figure 2B and C is due to the difference in growth conditions, as well as the concentration of cells present in the internalization assay (our unpublished data).
Several compounds in the sphingolipid synthesis pathway (Figure 1) could show rapid turnover because they are biosynthetic intermediates. In addition, several of these components, including phosphorylated sphingoid bases and ceramide, have been implicated in signaling events (Dickson and Lester, 1999; Mao et al., 1999). In order to identify which components of this pathway need to be synthesized for endocytosis, we combined the lcb1-100 mutation with other mutations that affect the utilization of exogenously added sphingoid bases. This allowed us to block endogenous sphingolipid biosynthesis upon temperature shift and control the metabolism of the exogenously added sphingoid bases in the same strain. Exogenously added DHS can be incorporated into ceramide and sphingolipid (Figures 1 and 2). Mutation of LCB3, encoding a sphingosine-1P phosphatase, reduces this incorporation drastically (Qie et al., 1997). YSR3 is homologous to LCB3, and can also dephosphorylate sphingosine-1P (Mao et al., 1997; Mao and Obeid, 2000). This suggests that the pathway to incorporate exogenous DHS into ceramide may require passage through a phosphorylated sphingoid base intermediate. Two sphingosine kinases have been identified, encoded by the LCB4 and LBC5 genes (Nagiec et al., 1998); however, it has not been determined whether they are required for synthesis of ceramide from exogenous DHS.
To test whether exogenously added DHS must be phosphorylated to make ceramide we constructed strains with multiple mutations. One strain could not synthesize sphingoid bases at 37°C, nor phosphorylate them (lcb1-100 lcb4 lcb5), and the other (lcb1-100 lcb3 ysr3) could not synthesize the bases at 37°C, nor dephosphorylate them. These strains were tested for their ability to make sphingolipids from exogenous [3H]DHS, because ceramides are rapidly converted into IPC, MIPC and M(IP)2C. To reproduce the conditions of the endocytosis assay, wild-type and mutant cells were preincubated for 15 min at 37°C, then [3H]DHS was added and incubation was continued for 15 min. Lipids were extracted and incorporation of [3H]DHS into sphingolipids was measured after TLC and visualization using a phosphorimager. In agreement with previous results (Mao et al., 1997; Qie et al., 1997; Mao and Obeid, 2000), mutation of the sphingosine-1P phosphatases completely blocked the synthesis of sphingolipids (>99% under our conditions), and therefore ceramides, from exogenous DHS (Figure 3, middle panel). In contrast to our expectations, there was no more accumulation of DHS-1P in this strain than in the lcb1-100 single mutant. This is due to the presence of the lcb1-100 mutation because an lcb3/ysr3 double mutant does synthesize higher levels of DHS-1P than the wild-type strain (our unpublished data). The heavy spot of [3H]DHS, which migrates further than IPC, is not shown. Two unidentified base-resistant spots (Y and Z) were consistently detected, but are not sphingolipids. The above results suggested that sphingoid base phosphorylation and dephosphorylation could be required for incorporation of exogenous DHS into ceramides.
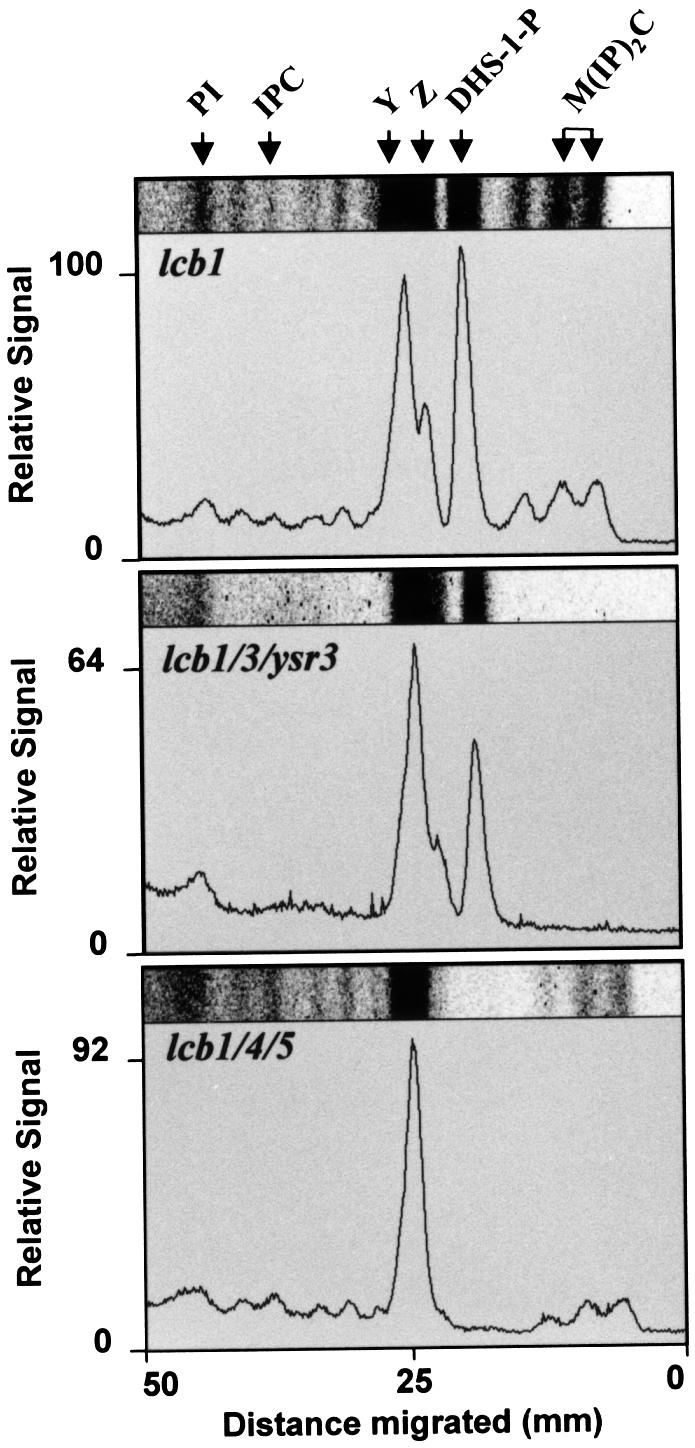
Fig. 3. Incorporation of [3H]DHS into sphingolipids and DHS-1P. The lcb1-100 (RH3809) or triple mutants (RH3859, lcb1/3/ ysr3 and RH4355, lcb1/4/5) were labeled with [3H]DHS at 37°C. Lipids were analyzed by TLC using solvent system II. Images of portions of individual lanes are shown and were scanned to quantify the incorporation. The incorporation of [3H]DHS into DHS-1P or sphingolipids in the lcb1-100 mutant was set to 100%. The positions of PI, IPC, DHS-1P, M(IP)2C, as well as two unknown products, Y and Z, are marked.
However, phosphorylation and dephosphorylation does not seem to be absolutely required for incorporation of exogenous DHS into sphingolipids because mutation of the sphingosine kinases (lcb4/5) only partially inhibited the incorporation of [3H]DHS into sphingolipids (Figure 3, lower panel; 45% of sphingolipid synthesis relative to lcb1-100). No [3H]DHS-1P (<1% of level in lcb1-100) was synthesized in the sphingosine kinase double mutant. [3H]DHS-1P synthesis was easily detected in the lcb1-100 mutant alone as well as in the sphingosine-1P phosphatase mutant (Figure 3). Therefore, it seems that the sphingosine-1P phosphatase may only be required for efficient incorporation of DHS into ceramides when the sphingosine kinase is present (see Discussion). This mutant did not accumulate spot Z, which could be PHS-1P according to its position and absence in the lcb1/4/5 mutant. However, we have not pursued this identification further. Uptake of [3H]DHS into cells (>95%) was approximately equal for all of the mutants tested.
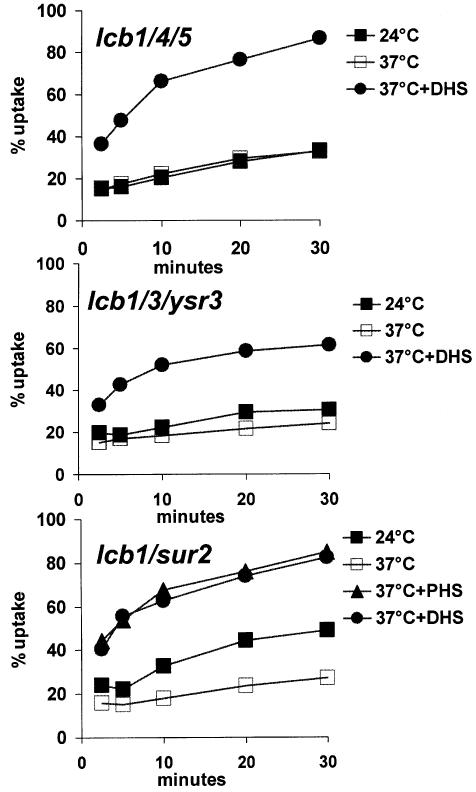
Since the lcb1/3/ysr3 mutant cannot make ceramides and the lcb1/4/5 cannot synthesize detectable amounts of DHS-1P from any source under our conditions we could next test whether these ceramides or DHS-1P are required for the restoration of endocytosis by exogenous DHS. We measured the internalization of α-factor in the triple mutant strains. At 24°C, the rates of endocytosis in the triple mutants were substantially lower than the lcb1-100 mutant alone (compare Figures 2B and 4; see Discussion). When we preincubated the triple mutants with DHS at 37°C and measured endocytosis, we found that DHS could still efficiently suppress the internalization defect in lcb1-100 cells devoid of sphingosine kinase activity (Figure 4). Furthermore, α-factor internalization is absolutely normal at 24 and 37°C in an lcb4/5 mutant strain lacking the lcb1-100 mutation (B.Zanolari, unpublished observations). These data show that synthesis of DHS-1P, which is not made under these conditions, is not required to suppress lcb1-100 internalization defect.
Fig. 4. Suppression of the α-factor internalization defect by DHS in mutants in sphingoid base metabolism. Strains RH4355 (lcb1/4/5), RH3859 (lcb1/3/ysr3) and RH4363 (lcb1/sur2) were assayed for [35S]α-factor internalization at 24 or at 37°C with or without exogenous DHS or PHS.
DHS also suppressed the lcb1-100 mutant incapable of dephosphorylating DHS-1P (lcb1/3/ysr3), although this suppression was somewhat less efficient (Figure 4). These results strongly suggest that ceramide and sphingolipids, which are not made under these conditions, are not required to suppress the lcb1-100 internalization defect. However, since the suppression seen in the lcb1/3/ysr3 mutant was somewhat weaker than the suppression seen in the sphingosine kinase mutant we sought an additional method to corroborate that ceramide is not required for the suppression.
Ceramide synthase can be potently inhibited by an antifungal compound, australifungin (Mandala et al., 1995). We first tested whether this compound is effective under the conditions of our endocytosis assays on the lcb1-100 mutant. Mutant cells were labeled with [3H]DHS as described above with the addition of 1 or 2 mg/l australifungin. Lipids were extracted, analyzed by TLC and visualized using a phosphorimager. Based on the disappearance of the sphingolipid spots, M(IP)2C and IPC, both concentrations of australifungin inhibited the synthesis of sphingolipids from exogenous [3H]DHS completely (Figure 5A). Next, we tested whether these concentrations of australifungin would have an effect on the suppression of the lcb1-100 mutant strain by DHS. Internalization of α-factor by lcb1-100 mutant cells was measured in the presence of australifungin. The inhibitor did not affect the ability of DHS to suppress the α-factor internalization defect in the lcb1-100 mutant (Figure 5B). Therefore, we conclude that ceramide synthesis is not required for the suppression of the internalization defect in the lcb1-100 mutant by DHS. The above results strongly point to the relevant lipid for internalization being a sphingoid base.
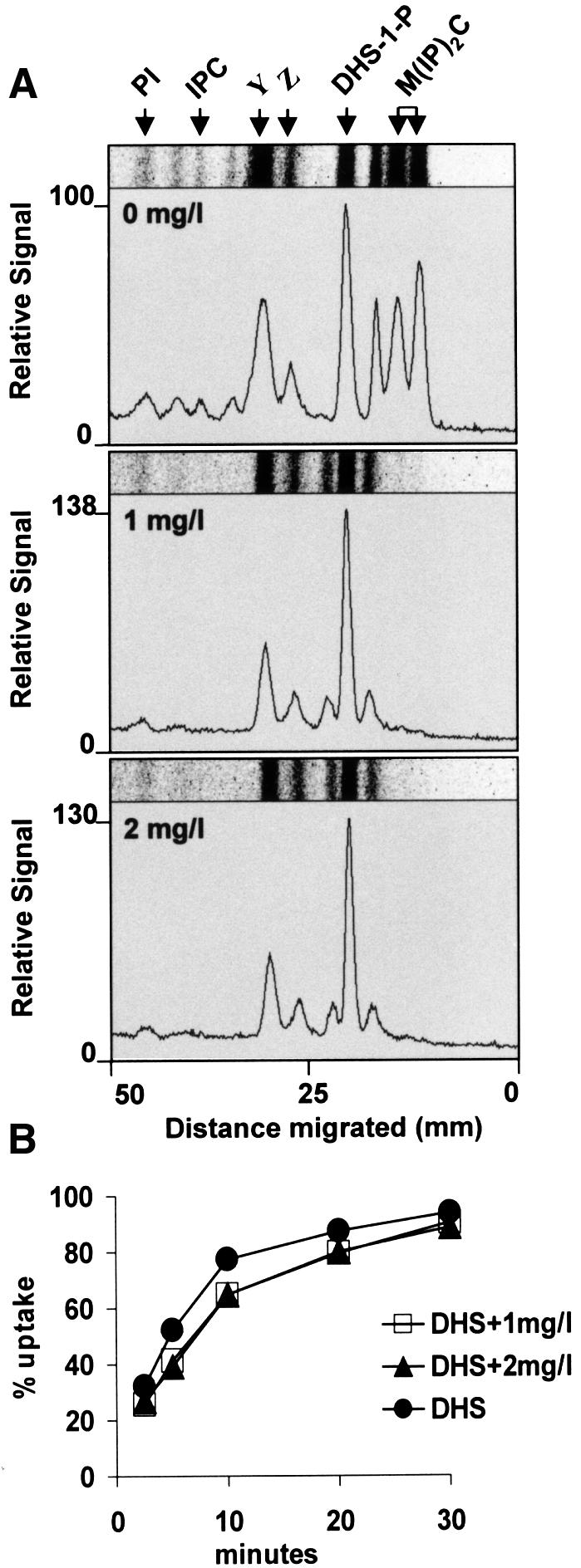
Fig. 5. Australifungin inhibits sphingolipid synthesis, but not endocytosis. (A) RH3809 cells were labeled with [3H]DHS at 37°C in the presence or absence of 1 or 2 mg/l australifungin and lipids were analyzed by TLC using solvent system II. Positions of PI, IPC, DHS-1P, M(IP)2C, and two unknown compounds, Y and Z, are marked. (B) Internalization of [35S]α-factor was measured in RH3809 cells in the presence of DHS and the presence or absence of australifungin at 37°C.
The two major sphingoid bases in Saccharomyces cerevisiae are DHS and PHS. DHS is converted to PHS by a hydroxylase, encoded by SUR2. Therefore, this step can be blocked in a sur2 mutant (Haak et al., 1997). We constructed a double mutant (lcb1-100 sur2) and tested whether DHS and PHS could suppress its internalization defect. Both DHS and PHS suppressed the internalization defect efficiently (Figure 4). Therefore, we conclude that either DHS or PHS can fulfill the rapid onset requirement for ceramide synthesis unveiled by analysis of the lcb1-100 mutant.
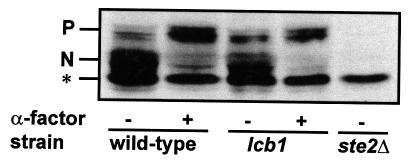
Sphingoid bases and ceramide have been postulated to stimulate specific kinases and phosphatases in yeast and other organisms (Huwiler et al., 1996; Nickels and Broach, 1996; Perry and Hannun, 1998). Since the α-factor receptor must be phosphorylated in order to be ubiquitylated and internalized (Hicke et al., 1998), we investigated whether receptor phosphorylation is one of the events that requires sphingoid base synthesis. Receptor phosphorylation is triggered by addition of α-factor to haploid Mata cells. Therefore, wild-type and lcb1-100 mutant cells were preshifted to 37°C for 15 min, treated with α-factor, protein extracts were prepared and analyzed by western blotting to detect the phosphorylation state of the receptor (Figure 6). When α-factor was added to wild-type cells, the α-factor receptor shifted to an apparently higher molecular weight. This is the typical behavior of this protein when phosphorylated (Hicke et al., 1998). The receptor underwent an identical shift in apparent mobility when the lcb1-100 cells were treated. Therefore, ongoing sphingoid base synthesis is not required for receptor phosphorylation.
Fig. 6. Phosphorylation of the α-factor receptor. RH1800 (wild-type), RH3809 (lcb1) or RH3162 (ste2Δ) cells were incubated in the presence or absence of α-factor at 37°C. Total proteins were extracted and the α-factor receptor revealed by western blotting. N, normal phosphorylated α-factor receptor seen in the absence of pheromone; P, mobility of hyperphosphorylated form of the α-factor receptor after pheromone treatment; *, non-specific band seen in ste2Δ mutant.
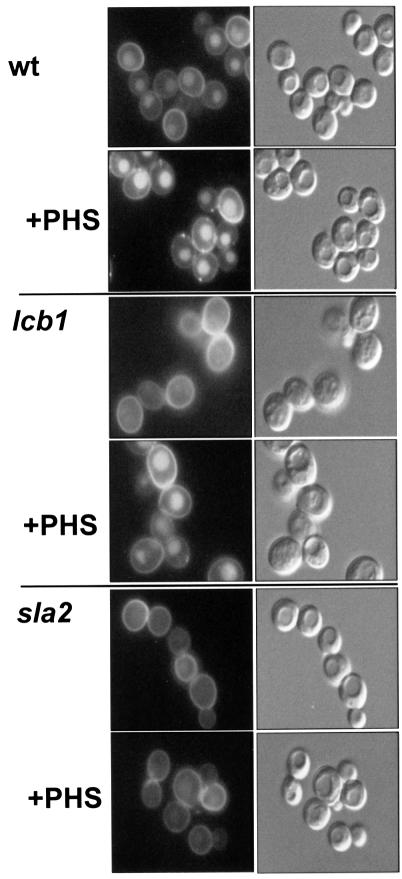
The lack of effect on receptor phosphorylation and the fact that accumulation of a non-specific endocytic marker, LY, is defective in lcb1-100 cells (Munn and Riezman, 1994) (Figure 7) suggest that the requirement for sphingoid base is general. Therefore, we tested whether PHS could suppress the defect in endocytosis revealed in the LY accumulation assay. Wild-type and lcb1-100 mutant cells were preshifted to 37°C in the presence or absence of PHS, then incubated with LY for 1 h. The cells were washed, examined by Nomarski optics and LY was visualized by fluorescence microscopy. Wild-type cells internalized LY into prominent vacuolar structures, visible as indentations with Nomarski optics. PHS addition stimulated LY accumulation in wild-type cells. The lcb1-100 mutant was defective in LY accumulation. One can also notice in the Nomarski picture that the vacuoles are somewhat fragmented. However, even when vacuoles can be seen clearly, they contain much less LY than wild-type vacuoles. When PHS was added to the mutant cells, LY accumulation was restored to normal even when certain vacuoles remained fragmented. Therefore, sphingoid base can restore the general endocytic defect of lcb1-100 cells. This restoration is specific to the lcb1-100 mutation, because PHS could not restore endocytosis to a temperature-sensitive sla2-41 (end4-1) mutant. These results show that sphingoid base is required generally for the internalization step of endocytosis.
Fig. 7. LY accumulation requires sphingoid base. RH1800 (wt), RH3809 (lcb1) and RH1597 (sla2) cells were incubated with LY at 37°C in the presence or absence of PHS. Cells were washed and visualized by fluorescence optics (left panels) or Nomarski optics (right panels).
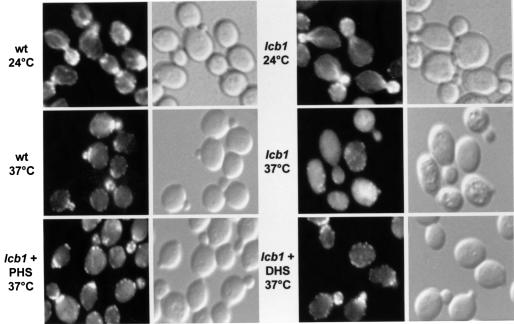
Actin plays a major role in the internalization step of endocytosis (Geli and Riezman, 1998; Wendland et al., 1998). Therefore, we analyzed the morphology of the actin cytoskeleton in wild-type and lcb1-100 mutant cells at 24 and 37°C. Cells were grown overnight at 24°C, shifted to 24 or 37°C for 2 h, fixed and stained with rhodamine–phalloidin to localize F-actin. Wild-type cells at both temperatures and lcb1-100 cells at 24°C showed a polarized actin distribution with a concentration of actin patches at sites of new cell growth (in small buds and at the septum preceding cell division). Only little cortical actin staining was seen in mother cells (Figure 8) (Kilmartin and Adams, 1984). In contrast, lcb1-100 mutant cells showed a non-polarized cortical actin staining at 37°C, indicating a defect in organization of the actin cytoskeleton in the absence of sphingoid base synthesis. Addition of PHS or DHS to the mutant cells partially restored proper actin localization. There was no apparent restoration of actin cables, but the general location of the cortical actin patches became more polarized, similar to the pattern observed in wild-type cells. Since sphingoid base could restore polarized actin staining, we conclude that at least one cause of the actin cytoskeleton defect in the lcb1-100 mutant is the lack of sphingoid base synthesis. This could explain why lcb1-100 mutant cells are defective for the internalization step of endocytosis.
Fig. 8. Actin staining in wild-type and mutant cells. RH1800 (wt) or RH3809 (lcb1) cells were incubated at 24 or at 37°C in the presence or absence of DHS or PHS, then fixed, and filamentous actin was visualized using TRITC–phalloidin (left panels) or Nomarski optics (right panels).
Discussion
The major finding of this study is that sphingoid base synthesis is required for the internalization step of endocytosis in S.cerevisiae. This discovery was made possible by the isolation and characterization of an endocytosis-deficient strain, the lcb1-100 mutant, which shows a temperature-sensitive serine palmitoyltransferase activity, the first step in sphingolipid biosynthesis. This mutant shows reduced sphingolipid synthesis at 24°C and a nearly complete block at 37°C, which allowed us to block the endogenous sphingolipid synthesis pathway rapidly and efficiently. Concomitantly, the mutant cells became defective for the internalization step of endocytosis. The rapid installation of this defect means that de novo synthesis of a lipid is necessary for the internalization step of endocytosis. This lipid may not be increased in amount after shift to 37°C or may be quickly depleted from cells or its site of action when its synthesis is stopped. Sphingolipids themselves were unlikely to be this product because they are very stable (Dickson and Lester, 1999). Sphingolipids are located at the plasma membrane, the proper location to play a role in the internalization step of endocytosis (Patton and Lester, 1991), but we know very little about their trafficking. However, we consider it unlikely that they could be rapidly depleted from the plasma membrane.
We showed that mutants that are unable to dephosphorylate phosphorylated sphingoid bases were unable to incorporate exogenous DHS into ceramide under the conditions of our assay because they could not make sphingolipids (Figure 3) from exogenous DHS. Since exogenous DHS could restore endocytosis under these conditions, this showed that the lipid synthesis requirement for endocytosis was not ceramide, nor a sphingolipid. This was further substantiated by showing that australifungin, an inhibitor of ceramide synthesis, blocked sphingolipid synthesis completely, but had almost no effect on the ability of exogenous DHS to suppress the lcb1-100 mutant. We also verified that ceramide was not made from exogenous DHS in these cells by measuring incorporation of radioactive DHS into ceramide (our unpublished data). Furthermore, preincubation of wild-type cells with australifungin (5 mg/l) for 15 min did not affect internalization of α-factor (our unpublished data). These results show that ceramide does not correspond to the lipid requirement uncovered by using the lcb1-100 mutant. This is important because ceramide has been suggested to be an important intracellular regulator and has been implicated in the regulation of phagocytosis (Grassme et al., 1997; Hinkovska-Galcheva et al., 1998). In addition, sphingomyelinase action on the external leaflet of plasma membranes, which generates ceramide, can induce ATP-independent endocytosis (Zha et al., 1998).
Our studies of exogenous DHS incorporation into sphingolipids has also shed some light upon the mechanism of utilization of exogenous long chain bases. As reported previously (Mao et al., 1997; Qie et al., 1997; Mao and Obeid, 2000), we found that mutations in sphingosine-1P phosphatases drastically reduced incorporation of exogenous DHS into sphingolipids. An hypothesis stemming from this finding is that exogenous DHS is phosphorylated in the cytosol and dephosphorylated at the endoplasmic reticulum for ceramide synthesis. Even though these events most likely do take place, they do not seem to be obligatory because disruption of the LCB4 and LCB5 genes encoding the sphingosine kinases did not block incorporation of exogenous DHS into sphingolipid completely. The above results could be explained in two ways. First, the sphingosine-1P phosphatases could have an additional function that is required for efficient incorporation of exogenous DHS into sphingolipids. Secondly, the phosphatases may only be required if the exogenous DHS becomes phosphorylated once inside the cell. We can rule out the first hypothesis because a mutant lacking both the sphingosine kinases and the major sphingosine-1P phosphatase (lcb1/3/4/5) incorporates exogenous DHS into sphingolipids as efficiently as the sphingosine kinase mutant alone (our unpublished data). Our measurements of incorporation of exogenous DHS into sphingolipids were performed under the same conditions in which we performed the endocytosis assays and the conclusions stated above might not hold for other conditions, such as long-term incubations.
Analysis of DHS suppression of the lcb1-100 mutation in sphingosine kinase mutant cells also allowed us to rule out a role for phosphorylated sphingoid base in internalization. Endocytosis was efficiently restored under conditions in which no detectable phosphorylated sphingoid bases were made. Phosphorylated sphingoid bases have been postulated to play a critical role in the stress response in yeast (Mandala et al., 1998; Skrzypek et al., 1999) and as second messengers in animal cells, in particular with respect to the cytoskeleton (Wang et al., 1997; Spiegel, 1999; Taylor et al., 1999). Apparently, they are not required for endocytosis in yeast. However, we cannot rule out the possibility that they modulate or stimulate endocytosis as part of the stress response. Some plasma membrane proteins, for example the uracil permease (Volland et al., 1994), are down-regulated in response to stress. In addition, sphingoid bases have been suggested to inhibit amino acid permeases (Skrzypek et al., 1998), perhaps through their down-regulation by endocytosis.
Our experiments have shown that the lipid that must be synthesized for endocytosis in the lcb1-100 mutant is not a phosphorylated sphingoid base, ceramide nor sphingolipids. We postulate that the relevant lipid is a sphingoid base, which can be either DHS or PHS, because either one seemed to be sufficient to suppress the double lcb1-100 sur2 mutant. To our knowledge, this is the first clear case for a physiological role of the synthesis of a sphingoid base, other than its normal function as an intermediate in ceramide or sphingosine-1P synthesis. We cannot formally rule out that the molecule required for endocytosis is an unknown metabolite of DHS or PHS. However, it should not require prior conversion to DHS-1P or PHS-1P by the sphingosine kinases.
Ongoing DHS or PHS synthesis seems to be an absolute requirement for the internalization step of endocytosis. This requirement is general because a non-specific marker is affected by the lcb1-100 mutation. It is specific because another endocytic mutant, sla2-41, is not suppressed by exogenous PHS. Therefore, the target for the function of DHS or PHS must affect the endocytic pathway in general. For this reason, we looked to the actin cytoskeleton, which has been implicated in the internalization step of the endocytic pathway. We found that sphingoid base is required for proper actin cytoskeleton morphology and thus, most likely, for proper actin cytoskeleton dynamics. The actin defects seen in the lcb1-100 mutant are temperature sensitive, like sphingoid base synthesis and can be at least partially restored by adding DHS back to the mutant cells, suggesting that they are caused by the block in sphingoid base synthesis. However, it is not possible to know from these types of studies whether the sphingoid base requirement is direct or not. The actin cytoskeleton undergoes a drastic change in morphology upon shift from 24 to 37°C in wild-type cells, which is a stress response, and polarized actin distribution is only restored after ∼1.5–2 h at 37°C. This long time period without sphingoid bases, ceramide and sphingolipid synthesis could have secondary effects. For this reason, we cannot be as certain about the precise lipid requirement for proper actin localization as we are for endocytosis, which can be measured very shortly after shift to non-permissive temperature. However, the suppression of the actin defect in lcb1-100 is specific, because the actin defect of sla2-41 (end4-1) (Wesp et al., 1997) was not suppressed at 37°C by DHS (our unpublished data).
How could a lipid, like a sphingoid base, act in the internalization step of endocytosis and regulation of the actin cytoskeleton? Since sphingoid bases are rapidly turned over they are ideal candidates to act as signaling molecules. In fact, a growing body of evidence suggests that sphingoid bases, including their phosphorylated derivatives, could play a signaling role in eukaryotic cells (Sadahira et al., 1992; Bornfeldt et al., 1995; Stam et al., 1998; Skrzypek et al., 1999). In the accompanying study (Friant et al., 2000), we present evidence that the function of sphingoid base synthesis in endocytosis and actin cytoskeleton organization is to control protein phosphorylation, suggesting a signaling role.
Even though we have clearly shown that sphingoid base synthesis is required for the internalization step of endocytosis, our data do not rule out a role for ceramide and/or sphingolipids in this process as well. We have been able to analyze a rapidly installed defect in endocytosis due to the loss of sphingoid base synthesis. For this rapid event, it is clear that ceramide and sphingolipids are not required. However, long-term depletion of these compounds could cause endocytic defects that we are not able to analyze. Indeed, we think that cell surface sphingolipids are likely to be required for endocytosis. One reason for this is that ergosterol is required for efficient endocytic uptake (Munn et al., 1999). Sphingolipids and sterols have been postulated to associate with each other in glycolipid enriched rafts (Harder and Simons, 1997) and have been shown to form domains in artificial lipid mixtures (Ahmed et al., 1997). A second, more empirical reason, is that lcb1-100 lcb3 ysr3 cells and lcb1-100 lcb4/5 cells, which have difficulty in converting exogenous DHS or PHS into sphingolipids, have lower rates of endocytosis at 24°C than the lcb1-100 mutant (Figures 2 and 4). Likewise, the endocytic internalization defect of the most strongly affected mutant cells, lcb1-100 lcb3 ysr3, is less well restored by DHS at 37°C than in lcb1-100 mutant cells (Figures 2 and 4). We suggest that these triple mutant cells have two endocytic internalization defects, one due to loss of sphingoid base, which is only seen strongly at 37°C, and a second defect, which is already present at 24°C, due to a lowered concentration of cell surface sphingolipids. Hopefully, this hypothesis can be addressed in the near future.
Materials and methods
Yeast strains and media
The yeast strains used in this study are shown in Table I. RH3809 and RH3804 (MATα lcb1-100 leu2 ura3 lys2 trp1 bar1-1) were obtained by additional backcrosses of RH2608 (Munn and Riezman, 1994) to RH1800. Strain YSR2 2-1D (MATα lcb3Δ::KanMx ysr3Δ::URA3 ura3 leu2 trp1 HMLa) (Mao et al., 1997) was backcrossed twice in our strain background to generate RH3859. The deletion/insertion alleles sur2::LEU2, lcb4::URA3, lcb5::LEU2 were generated by transformation of RH1800 with PCR products (Wach, 1996). The entire open reading frames were deleted and replaced with the designated genes. Disruptions were confirmed by PCR. Disruptants were then crossed to RH3804 to generate RH4355 and RH4363. Yeast cells were grown overnight in semi-synthetic medium, SDYE (Horvath et al., 1994), or when indicated, in rich medium, YPUAD (Dulic et al., 1991).
Table I. MATa yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| RH1800 | leu2 his4 ura3 bar1-1 | Raths et al. (1993) |
| RH1597 | sla2-41 leu2 his4 ura3 bar1-1 | Raths et al. (1993) |
| RH3162 | ste2::LEU2 leu2 his3 trp1 bar1-1 | Hicke and Riezman (1996) |
| RH3809 | lcb1-100 leu2 his4 ura3 bar1-1 | this study |
| RH3859 | lcb1-100 lcb3Δ::KanMx ysr3Δ::URA3 leu2 his4 ura3 bar1-1 | this study |
| RH4355 | lcb1-100 lcb4::URA3 lcb5::LEU2 leu2 his4 ura3 lys2 bar1-1 | this study |
| RH4363 | lcb1-100 sur2::LEU2 leu2 his4 ura3 bar1-1 | this study |
Labeling with [3H]DHS and [3H]myo-inositol
Cells were grown overnight in SDYE at 24°C to log phase. From that culture 2 × 108 cells (Figure 3) or 1 × 108 cells (Figures 2 and 5) were harvested and washed three times with SD medium (Dulic et al., 1991) for labeling with [3H]DHS, or SD medium without inositol for labeling with [3H]myo-inositol. The cells were then resuspended in 0.5 ml of SD or SD without inositol and preincubated for 15 min at 37°C. The labeling was then initiated by adding 25 µCi of [3H]myo-inositol (10–25 Ci/mmol in aqueous solution; NEN Life Science Products, Inc., Boston, MA) or 4 µCi of [4,5-3H]d-erythro-dihydrosphingosine ([3H]DHS) in ethanol (60 Ci/mmol; American Radiolabeled Chemical Inc., St Louis, MO) and incubation was continued for 15 min at 37°C with shaking. If present, DHS was added at the start of the preincubation and australifungin (provided by Merck, USA) (in ethanol) was added just before the labeling. The incubation was stopped by adding NaF and NaN3 to a final concentration of 10 mM. The cells were then washed with cold water, and the lipids were extracted by resuspending the cells in 500 µl chloroform–methanol–water (CMW, 10:10:3) and mixed by vortexing with glass beads. The organic phase was collected after centrifuging for 5 min at 13 000 g, and the pellet was re-extracted with 500 µl of CMW. The pooled lipid extracts were dried under nitrogen and dissolved in 100 µl of H2O-saturated n-butanol. The butanol was extracted with 50 µl of water. The aqueous phase was back-extracted twice with 100 µl of H2O-saturated n-butanol. The combined butanol phase was dried under nitrogen. The lipids were dissolved in CMW for TLC on Kieselgel 60 plates (20 × 20; Merck, Darmstadt, Germany) and developed in solvent system I [chloroform–methanol–0.25% KCl (55:45:10)] or solvent system II [chloroform–methanol–4.2 N ammonium hydroxide (9:7:2)]. Radiolabeled lipids were visualized and quantified on a Cyclone Storage Phosphor System by using a tritium-sensitive screen (Packard, Meriden, CT). The lipids, phosphatidylinositol (PI), IPC, DHS-1P, MIPC, lysophosphatidylinositol (lysoPI), and M(IP)2C were identified by using [3H]inositol-radiolabeled lipids and [3H]DHS-1P (prepared by incubating [3H]DHS with yeast cytosol and ATP-regenerating system) as standards, and by chemical treatment of the lipids by mild alkaline hydrolysis with NaOH (Conzelmann et al., 1992) and cleavage with phosphatidylinositol specific phospholipase C (our unpublished data).
Endocytosis assays
LY uptake was performed on cells grown overnight in YPUAD as described previously (Dulic et al., 1991). Cells were preincubated at 37°C for 15 min prior to adding LY. PHS (50 µM) was added during this preincubation where indicated. α-factor internalization assays were performed according to the continuous presence protocol (Dulic et al., 1991) with a 15 min preincubation at the indicated temperature before addition of [35S]α-factor to cells grown in SDYE and resuspended to 2 × 108 cells/ml in SDYE. For the experiments described in Figure 2C, cells were grown overnight in YPUAD and [35S]α-factor internalization was performed on cells at 5 × 108 cells/ml in SDYE. DHS (Matreya) or PHS (Sigma) was added to 50 µM at the start of the preincubation. Internalized α-factor was measured by its resistance to incubation and washes with pH 1.1 buffer. All assays were performed two or more times and the differences between the individual time points was <10%.
Receptor modification
Phosphorylation of the α-factor receptor was determined as described previously (Hicke et al., 1998). Cells were grown overnight in YPUAD and resuspended in prewarmed 37°C YPUAD. After 5 min incubation, cycloheximide (10 µg/ml) was added and incubation continued for 10 min. Unlabeled α-factor (Zanolari and Riezman, 1991; 10–7 M) was added and incubation continued for 8 min. Ste2p was extracted from the cells and analyzed by western blotting as described.
Actin staining
Cells were grown to early log phase in YPUAD, then were washed and resuspended in YPUAD at 1 × 107 cells/ml. Cells were incubated for 15 min at 24 or 37°C before adding PHS (50 µM) or DHS (50 µM). Incubation was continued for 1 h before fixation in formaldehyde and staining with TRITC–phalloidin (Sigma, St Louis, MO) to visualize F-actin essentially as described previously (Bénédetti et al., 1994).
Acknowledgments
Acknowledgements
We would like to thank Merck for providing australifungin, R.Lester, L.Obeid, Y.Hannun for yeast strains, Jeannette Holenstein and Thomas Aust for technical assistance, and A.Heese-Peck and P.Morsomme for comments improving this manuscript. This work was supported by the University of Basel, EMBO and HFSP long-term fellowships (to S.F.) and a grant from the Swiss National Science Foundation (to H.R.).
References
- Ahmed S.N., Brown,D.A. and London,E. (1997) On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry, 36, 10944–10953. [DOI] [PubMed] [Google Scholar]
- Alb J.G. Jr, Kearns,M.A. and Bankaitis,V.A. (1996) Phospholipid metabolism and membrane dynamics. Curr. Opin. Cell Biol., 8, 534–541. [DOI] [PubMed] [Google Scholar]
- Bénédetti H., Raths,S., Crausaz,F. and Riezman,H. (1994) The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell, 5, 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K.E. et al. (1995) Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol., 130, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buede R., Rinker-Schaffer,C., Pinto,W.J., Lester,R.L. and Dickson,R.C. (1991) Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J. Bacteriol., 173, 4325–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Puoti,A., Lester,R.L. and Desponds,C. (1992) Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J., 11, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P. and Letourneur,F. (1997) Coatomer (COPI)-coated vesicles: role in intracellular transport and protein sorting. Curr. Opin. Cell Biol., 9, 484–487. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Emr,S.D., McPherson,P.S. and Novick,P. (1996) Phosphoinositides as regulators in membrane traffic. Science, 271, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Desrivières S., Cooke,F.T., Parker,P.J. and Hall,M.N. (1998) MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem., 273, 15787–15793. [DOI] [PubMed] [Google Scholar]
- Dickson R.C. and Lester,R.L. (1999) Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta, 1438, 305–321. [DOI] [PubMed] [Google Scholar]
- Dulic V., Egerton,M., Elguindi,I., Raths,S., Singer,B. and Riezman,H. (1991) Yeast endocytosis assays. Methods Enzymol., 194, 697–710. [DOI] [PubMed] [Google Scholar]
- Friant S., Zanolari,B. and Riezman,H. (2000) Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J., 19, 2834–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J.M., Moreau,V., Andre,B., Volland,C. and Haguenauer-Tsapis,R. (1996) Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin–protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem., 271, 10946–10952. [DOI] [PubMed] [Google Scholar]
- Geli M.I. and Riezman,H. (1998) Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci., 111, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P.J., Machesky,L.M., Baldassare,J.J. and Pollard,T.D. (1990) The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science, 247, 1575–1578. [DOI] [PubMed] [Google Scholar]
- Grassme H., Gulbins,E., Brenner,B., Ferlinz,K., Sandhoff,K., Harzer,K., Lang,F. and Meyer,T.F. (1997) Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell, 91, 605–615. [DOI] [PubMed] [Google Scholar]
- Haak D., Gable,K., Beeler,T. and Dunn,T. (1997) Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem., 272, 29704–29710. [DOI] [PubMed] [Google Scholar]
- Haffner C., Takei,K., Chen,H., Ringstad,N., Hudson,A., Butler,M.H., Salcini,A.E., Di Fiore,P.P. and De Camilli,P. (1997) Synaptojanin 1: localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett., 419, 175–180. [DOI] [PubMed] [Google Scholar]
- Harder T. and Simons,K. (1997) Caveolae, DIGs and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol., 9, 534–542. [DOI] [PubMed] [Google Scholar]
- He H. et al. (1998) Role of phosphatidylinositol 4,5-bisphosphate in Ras/Rac-induced disruption of the cortactin–actomyosin II complex and malignant transformation. Mol. Cell. Biol., 18, 3829–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. (1999) Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol., 9, 107–112. [DOI] [PubMed] [Google Scholar]
- Hicke L. and Riezman,H. (1996) Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell, 84, 277–287. [DOI] [PubMed] [Google Scholar]
- Hicke L., Zanolari,B. and Riezman,H. (1998) Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J. Cell Biol., 141, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkovska-Galcheva V., Kjeldsen,L., Mansfield,P.J., Boxer,L.A., Shayman,J.A. and Suchard,S.J. (1998) Activation of a plasma membrane-associated neutral sphingomyelinase and concomitant ceramide accumulation during IgG-dependent phagocytosis in human polymorphonuclear leukocytes. Blood, 91, 4761–4769. [PubMed] [Google Scholar]
- Holtzman D.A., Yang,S. and Drubin,D.G. (1993) Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J. Cell Biol., 122, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K., Terui,S., Minemura,M., Qadota,H., Anraku,Y., Kanaho,Y. and Ohya,Y. (1998) Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J. Biol. Chem., 273, 15779–15786. [DOI] [PubMed] [Google Scholar]
- Horvath A., Sütterlin,C., Manning-Krieg,U., Movva,N.R. and Riezman,H. (1994) Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J., 13, 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A., Brunner,J., Hummel,R., Vervoordeldonk,M., Stabel,S., van den Bosch,H. and Pfeilschifter,J. (1996) Ceramide-binding and activation defines protein kinase c-Raf as a ceramide-activated protein kinase. Proc. Natl Acad. Sci. USA, 93, 6959–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G.M., Richards,A., Wahl,T., Mao,C., Obeid,L. and Hannun,Y. (1997) Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem., 272, 32566–32572. [DOI] [PubMed] [Google Scholar]
- Kearns B.G., McGee,T.P., Mayinger,P., Gedvilaite,A., Phillips,S.E., Kagiwada,S. and Bankaitis,V.A. (1997) Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature, 387, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J.V. and Adams,A.E. (1984) Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol., 98, 922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Bonifacino,J.S. and Riezman,H. (1997) Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol., 9, 488–495. [DOI] [PubMed] [Google Scholar]
- Kübler E. and Riezman,H. (1993) Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J., 12, 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M.J. and Schekman,R. (1997) COPII and secretory cargo capture into transport vesicles. Curr. Opin. Cell Biol., 9, 477–483. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Fujimoto,L.M., Yin,H.L. and Schmid,S.L. (1997) The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem., 272, 20332–20335. [DOI] [PubMed] [Google Scholar]
- Mandala S.M. et al. (1995) The discovery of australifungin, a novel inhibitor of sphinganine N-acyltransferase from Sporormiella australis. Producing organism, fermentation, isolation and biological activity. J. Antibiot., 48, 349–356. [DOI] [PubMed] [Google Scholar]
- Mandala S.M., Thornton,R., Tu,Z., Kurtz,M.B., Nickels,J., Broach,J., Menzeleev,R. and Spiegel,S. (1998) Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl Acad. Sci. USA, 95, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C. and Obeid,L.M. (2000) Yeast sphingosine-1-phosphate phosphatases: assay, expression, deletion, purification and cellular localization by GFP tagging. Methods Enzymol., 311, 223–232. [DOI] [PubMed] [Google Scholar]
- Mao C., Wadleigh,M., Jenkins,G.M., Hannun,Y.A. and Obeid,L.M. (1997) Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J. Biol. Chem., 272, 28690–28694. [DOI] [PubMed] [Google Scholar]
- Mao C., Saba,J.D. and Obeid,L.M. (1999) The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochem. J., 342, 667–675. [PMC free article] [PubMed] [Google Scholar]
- Martin T.F. and Kowalchyk,J.A. (1997) Docked secretory vesicles undergo Ca2+-activated exocytosis in a cell-free system. J. Biol. Chem., 272, 14447–14453. [DOI] [PubMed] [Google Scholar]
- Munn A.L. and Riezman,H. (1994) Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J. Cell Biol., 127, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L., Stevenson,B.J., Geli,M.I. and Riezman,H. (1995) end5, end6 and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell, 6, 1721–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L., Heese-Peck,A., Stevenson,B.J., Pichler,H. and Riezman,H. (1999) Specific sterols required for the internalization step of endocytosis in yeast. Mol. Biol. Cell, 10, 3943–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec M.M., Skrzypek,M., Nagiec,E.E., Lester,R.L. and Dickson,R.C. (1998) The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases. J. Biol. Chem., 273, 19437–19442. [DOI] [PubMed] [Google Scholar]
- Nickels J.T. and Broach,J.R. (1996) A ceramide-activated protein phosphatase mediates ceramide-induced G1 arrest of Saccharomyces cerevisiae. Genes Dev., 10, 382–394. [DOI] [PubMed] [Google Scholar]
- Novick P. and Zerial,M. (1997) The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol., 9, 496–504. [DOI] [PubMed] [Google Scholar]
- Patton J.L. and Lester,R.L. (1991) The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J. Bacteriol., 173, 3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D.K. and Hannun,Y.A. (1998) The role of ceramide in cell signaling. Biochim. Biophys. Acta, 1436, 233–243. [DOI] [PubMed] [Google Scholar]
- Qie L., Nagiec,M.M., Baltisberger,J.A., Lester,R.L. and Dickson,R.C. (1997) Identification of a Saccharomyces gene, LCB3, necessary for incorporation of exogenous long chain bases into sphingolipids. J. Biol. Chem., 272, 16110–16117. [DOI] [PubMed] [Google Scholar]
- Rapoport I., Miyazaki,M., Boll,W., Duckworth,B., Cantley,L.C., Shoelson,S. and Kirchhausen,T. (1997) Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J., 16, 2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S., Rohrer,J., Crausaz,F. and Riezman,H. (1993) end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol., 120, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal S.K., Skretting,G., Garred,O., Vilhardt,F., van Deurs,B. and Sandvig,K. (1999) Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell, 10, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. (1996) The protein machinery of vesicle budding and fusion. Protein Sci., 5, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadahira Y., Ruan,F., Hakomori,S. and Igarashi,Y. (1992) Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc. Natl Acad. Sci. USA, 89, 9686–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T., Itoh,T., Miura,K. and Takenawa,T. (1997) Phosphatidylinositol 4,5-bisphosphate phosphatase regulates the rearrangement of actin filaments. Mol. Cell. Biol., 17, 3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. and Hall,M.N. (1998) Signaling to the actin cytoskeleton. Annu. Rev. Cell. Dev. Biol., 14, 305–338. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Wolde,M., Thiele,C., Fest,W., Kratzin,H., Podtelejnikov,A.V., Witke,W., Huttner,W.B. and Soling,H.D. (1999) Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature, 401, 133–141. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B., Nemoto,Y., Daniell,L., Ferro-Novick,S. and De Camilli,P. (1998) Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J. Cell Sci., 111, 3347–3356. [DOI] [PubMed] [Google Scholar]
- Skrzypek M.S., Nagiec,M.M., Lester,R.L. and Dickson,R.C. (1998) Inhibition of amino acid transport by sphingoid long chain bases in Saccharomyces cerevisiae. J. Biol. Chem., 273, 2829–2834. [DOI] [PubMed] [Google Scholar]
- Skrzypek M.S., Nagiec,M.M., Lester,R.L. and Dickson,R.C. (1999) Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol., 181, 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S. (1999) Sphingosine 1-phosphate: a prototype of a new class of second messengers. J. Leukoc. Biol., 65, 341–344. [DOI] [PubMed] [Google Scholar]
- Stam J.C., Michiels,F., van der Kammen,R.A., Moolenaar,W.H. and Collard,J.G. (1998) Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J., 17, 4066–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.M., Macklem,M.M. and Parsons,J.T. (1999) Cytoskeletal changes induced by GRAF, the GTPase regulator associated with focal adhesion kinase, are mediated by Rho. J. Cell Sci., 112, 231–242. [DOI] [PubMed] [Google Scholar]
- Thiele C., Hannah,M.J., Fahrenholz,F. and Huttner,W.B. (2000) Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nature Cell Biol., 2, 42–49. [DOI] [PubMed] [Google Scholar]
- Volland C., Urban-Grimal,D., Geraud,G. and Haguenauer-Tsapis,R. (1994) Endocytosis and degradation of the yeast uracil permease under adverse conditions. J. Biol. Chem., 269, 9833–9841. [PubMed] [Google Scholar]
- Wach A. (1996) PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S.cerevisiae. Yeast, 12, 259–265. [DOI] [PubMed] [Google Scholar]
- Wang F., Nobes,C.D., Hall,A. and Spiegel,S. (1997) Sphingosine 1-phosphate stimulates rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem. J., 324, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.B., Dickson,R.C. and Lester,R.L. (1998) Heat-induced elevation of ceramide in Saccharomyces cerevisiae via de novo synthesis. J. Biol. Chem., 273, 7235–7243. [DOI] [PubMed] [Google Scholar]
- Wendland B., McCaffery,J.M., Xiao,Q. and Emr,S.D. (1996) A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J. Cell Biol., 135, 1485–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B., Emr,S.D. and Riezman,H. (1998) Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol., 10, 513–522. [DOI] [PubMed] [Google Scholar]
- Wesp A., Hicke,L., Palecek,J., Lombardi,R., Aust,T., Munn,A.L. and Riezman,H. (1997) End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell, 8, 2291–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari B. and Riezman,H. (1991) Quantitation of α-factor internalization and response during the Saccharomyces cerevisiae cell cycle. Mol. Cell. Biol., 11, 5251–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X., Pierini,L.M., Leopold,P.L., Skiba,P.J., Tabas,I. and Maxfield,F.R. (1998) Sphingomyelinase treatment induces ATP-independent endocytosis. J. Cell Biol., 140, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]