Abstract
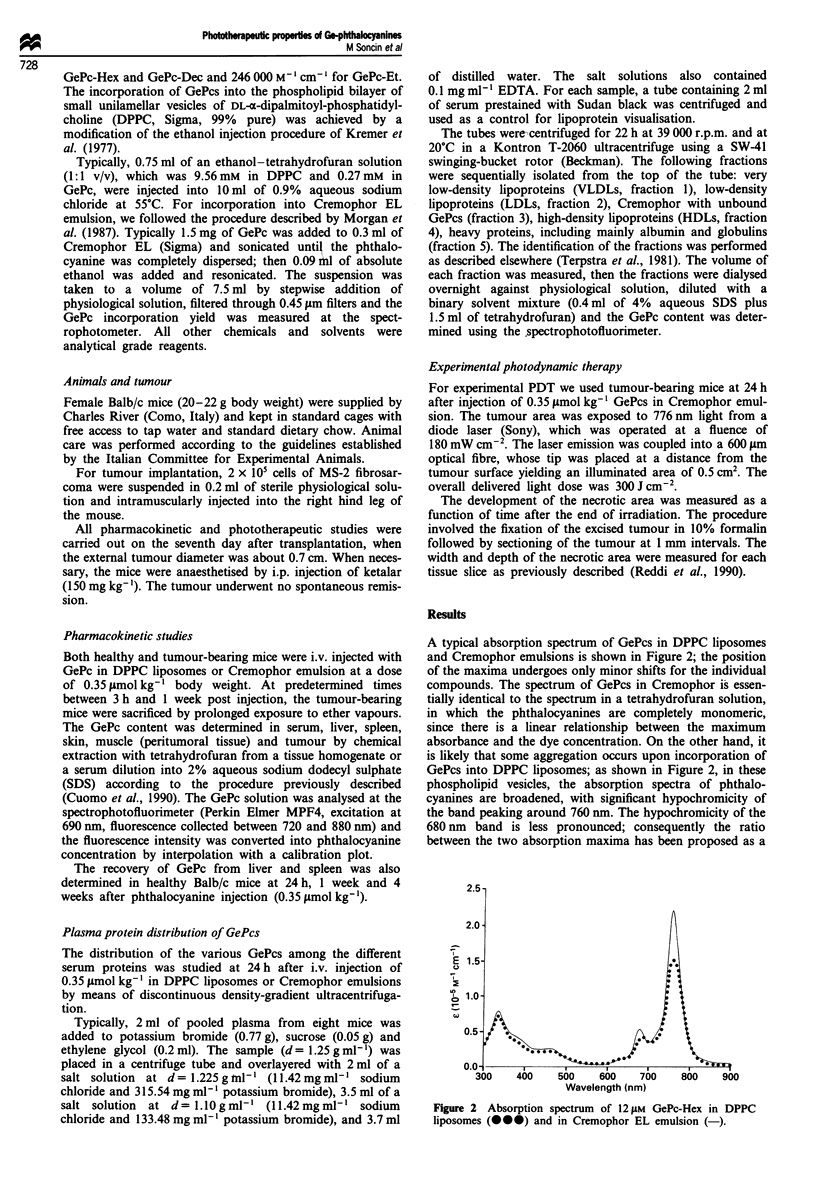
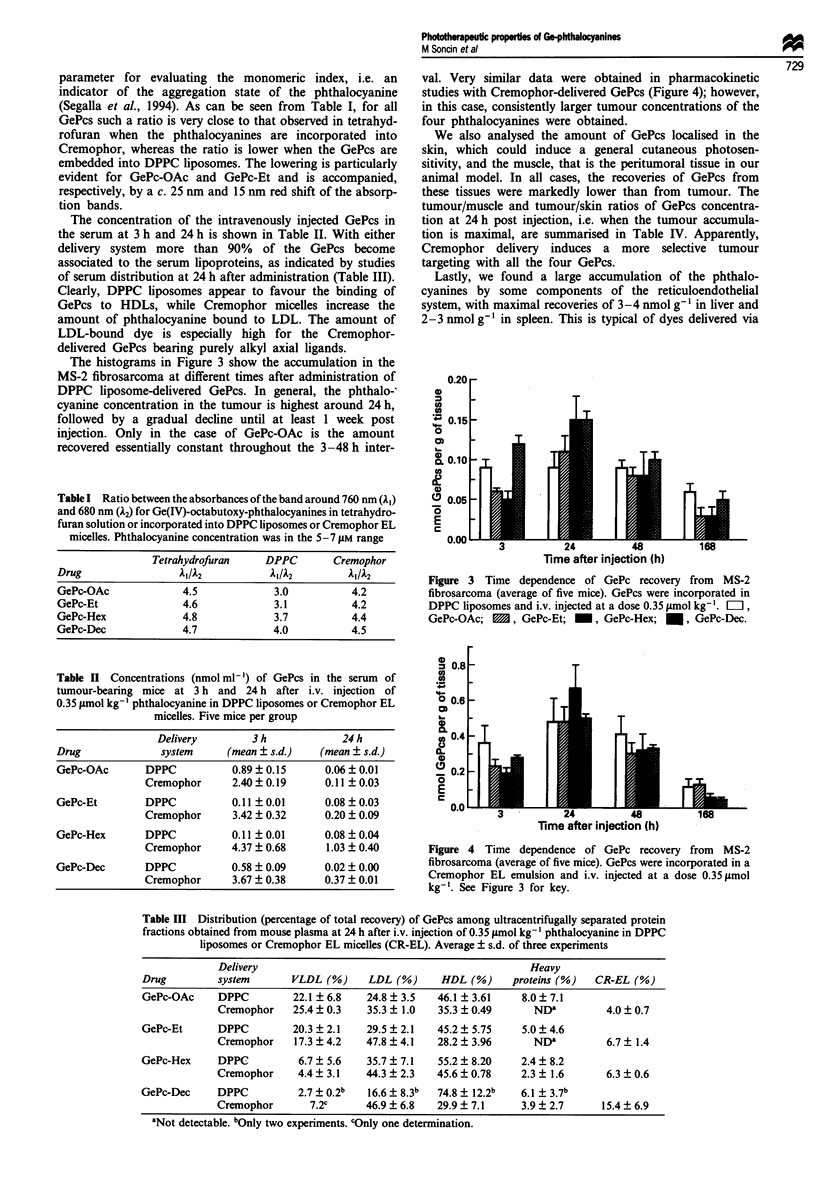
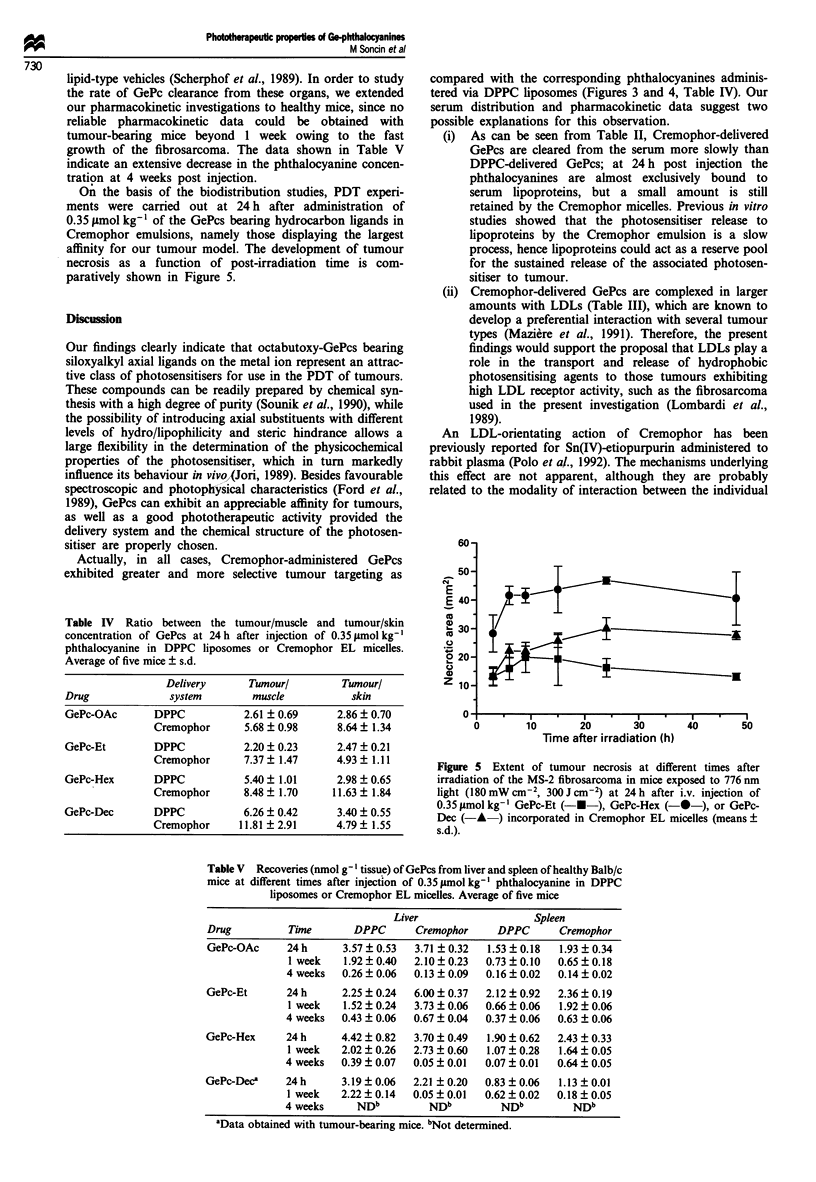
Four Ge(IV)-octabutoxy-phthalocyanines (GePcs) bearing two alkyl-type axial ligands were assayed for their pharmacokinetic properties and phototherapeutic efficiency in Balb/c mice bearing an intramuscularly transplanted MS-2 fibrosarcoma. The GePcs were i.v. injected at a dose of 0.35 mumol kg-1 body weight after incorporation into either Cremophor emulsions or small unilamellar liposomes of dipalmitoyl-phosphatidylcholine (DPPC). Both the nature of the delivery system and the chemical structure of the phthalocyanine were found to affect the behaviour of the GePcs in vivo. Thus, Cremophor-administered GePcs invariably yielded a more prolonged serum retention and a larger association with low-density lipoproteins (LDLs) as compared with the corresponding liposome-delivered phthalocyanines. This led to a greater efficiency and selectivity of tumour targeting. These effects were more pronounced for those GePcs having relatively long alkyl chains (hexyl to decyl) in the axial ligands. Maximal tumour accumulation (0.67 nmol per g of tissue) was found for Ge-Pc(hexyl)2 at 24 h after injection. Consistently, the Ge-Pc(hexyl)2, administered via Cremophor, showed the highest phototherapeutic activity towards MS-2 fibrosarcoma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellemo C., Jori G., Rihter B. D., Kenney M. E., Rodgers M. A. Si(IV)-naphthalocyanine: modulation of its pharmacokinetic properties through the use of hydrophilic axial ligands. Cancer Lett. 1992 Aug 14;65(2):145–150. doi: 10.1016/0304-3835(92)90159-s. [DOI] [PubMed] [Google Scholar]
- Bellnier D. A., Ho Y. K., Pandey R. K., Missert J. R., Dougherty T. J. Distribution and elimination of Photofrin II in mice. Photochem Photobiol. 1989 Aug;50(2):221–228. doi: 10.1111/j.1751-1097.1989.tb04152.x. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Marshall J. F., Svensen R., Bedwell J., Hart I. R. Effect of sulfonation on the cell and tissue distribution of the photosensitizer aluminum phthalocyanine. Cancer Res. 1990 Aug 1;50(15):4533–4538. [PubMed] [Google Scholar]
- Cuomo V., Jori G., Rihter B., Kenney M. E., Rodgers M. A. Liposome-delivered Si(IV)-naphthalocyanine as a photodynamic sensitiser for experimental tumours: pharmacokinetic and phototherapeutic studies. Br J Cancer. 1990 Dec;62(6):966–970. doi: 10.1038/bjc.1990.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo V., Jori G., Rihter B., Kenney M. E., Rodgers M. A. Tumour-localising and -photosensitizing properties of liposome-delivered Ge(IV)-octabutoxy-phthalocyanine. Br J Cancer. 1991 Jul;64(1):93–95. doi: 10.1038/bjc.1991.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy. Photochem Photobiol. 1993 Dec;58(6):895–900. doi: 10.1111/j.1751-1097.1993.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 1987 Jun;45(6):879–889. doi: 10.1111/j.1751-1097.1987.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Evensen J. F., Sommer S., Rimington C., Moan J. Photodynamic therapy of C3H mouse mammary carcinoma with haematoporphyrin di-ethers as sensitizers. Br J Cancer. 1987 May;55(5):483–486. doi: 10.1038/bjc.1987.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. E., Rihter B. D., Kenney M. E., Rodgers M. A. Photoproperties of alkoxy-substituted phthalocyanines with deep-red optical absorbance. Photochem Photobiol. 1989 Sep;50(3):277–282. doi: 10.1111/j.1751-1097.1989.tb04160.x. [DOI] [PubMed] [Google Scholar]
- Jori G. In vivo transport and pharmacokinetic behavior of tumour photosensitizers. Ciba Found Symp. 1989;146:78–94. doi: 10.1002/9780470513842.ch6. [DOI] [PubMed] [Google Scholar]
- Kongshaug M., Cheng L. S., Moan J., Rimington C. Interaction of cremophor EL with human plasma. Int J Biochem. 1991;23(4):473–478. doi: 10.1016/0020-711x(91)90176-n. [DOI] [PubMed] [Google Scholar]
- Kongshaug M. Distribution of tetrapyrrole photosensitizers among human plasma proteins. Int J Biochem. 1992 Aug;24(8):1239–1265. doi: 10.1016/0020-711x(92)90200-k. [DOI] [PubMed] [Google Scholar]
- Korbelik M. Distribution of disulfonated and tetrasulfonated aluminum phthalocyanine between malignant and host cell populations of a murine fibrosarcoma. J Photochem Photobiol B. 1993 Oct;20(2-3):173–181. doi: 10.1016/1011-1344(93)80148-3. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Esker M. W., Pathmamanoharan C., Wiersema P. H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977 Aug 23;16(17):3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- Lombardi P., Norata G., Maggi F. M., Canti G., Franco P., Nicolin A., Catapano A. L. Assimilation of LDL by experimental tumours in mice. Biochim Biophys Acta. 1989 Jun 28;1003(3):301–306. doi: 10.1016/0005-2760(89)90236-1. [DOI] [PubMed] [Google Scholar]
- Mazière J. C., Morlière P., Santus R. The role of the low density lipoprotein receptor pathway in the delivery of lipophilic photosensitizers in the photodynamic therapy of tumours. J Photochem Photobiol B. 1991 Mar;8(4):351–360. doi: 10.1016/1011-1344(91)80111-t. [DOI] [PubMed] [Google Scholar]
- Milanesi C., Biolo R., Reddi E., Jori G. Ultrastructural studies on the mechanism of the photodynamic therapy of tumors. Photochem Photobiol. 1987 Nov;46(5):675–681. doi: 10.1111/j.1751-1097.1987.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Moan J., Berg K. Photochemotherapy of cancer: experimental research. Photochem Photobiol. 1992 Jun;55(6):931–948. doi: 10.1111/j.1751-1097.1992.tb08541.x. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Garbo G. M., Kreimer-Birnbaum M., Keck R. W., Chaudhuri K., Selman S. H. Morphological study of the combined effect of purpurin derivatives and light on transplantable rat bladder tumors. Cancer Res. 1987 Jan 15;47(2):496–498. [PubMed] [Google Scholar]
- Polo L., Reddi E., Garbo G. M., Morgan A. R., Jori G. The distribution of the tumour photosensitizers Zn(II)-phthalocyanine and Sn(IV)-etiopurpurin among rabbit plasma proteins. Cancer Lett. 1992 Oct 21;66(3):217–223. doi: 10.1016/0304-3835(92)90250-y. [DOI] [PubMed] [Google Scholar]
- Reddi E., Zhou C., Biolo R., Menegaldo E., Jori G. Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br J Cancer. 1990 Mar;61(3):407–411. doi: 10.1038/bjc.1990.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherphof G. L., Spanjer H. H., Derksen J. T., Lázár G., Roerdink F. H. Targeting of liposomes to liver cells. Horiz Biochem Biophys. 1989;9:281–291. [PubMed] [Google Scholar]
- Segalla A., Milanesi C., Jori G., Capraro H. G., Isele U., Schieweck K. CGP 55398, a liposomal Ge(IV) phthalocyanine bearing two axially ligated cholesterol moieties: a new potential agent for photodynamic therapy of tumours. Br J Cancer. 1994 May;69(5):817–825. doi: 10.1038/bjc.1994.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert L. M., Novik B. R., Johnson P. The use of donated oocytes for the treatment of infertility. N C Med J. 1994 Oct;55(10):483–486. [PubMed] [Google Scholar]
- Terpstra A. H., Woodward C. J., Sanchez-Muniz F. J. Improved techniques for the separation of serum lipoproteins by density gradient ultracentrifugation: visualization by prestaining and rapid separation of serum lipoproteins from small volumes of serum. Anal Biochem. 1981 Feb;111(1):149–157. doi: 10.1016/0003-2697(81)90243-8. [DOI] [PubMed] [Google Scholar]
- Tralau C. J., Barr H., Sandeman D. R., Barton T., Lewin M. R., Bown S. G. Aluminum sulfonated phthalocyanine distribution in rodent tumors of the colon, brain and pancreas. Photochem Photobiol. 1987 Nov;46(5):777–781. doi: 10.1111/j.1751-1097.1987.tb04847.x. [DOI] [PubMed] [Google Scholar]


