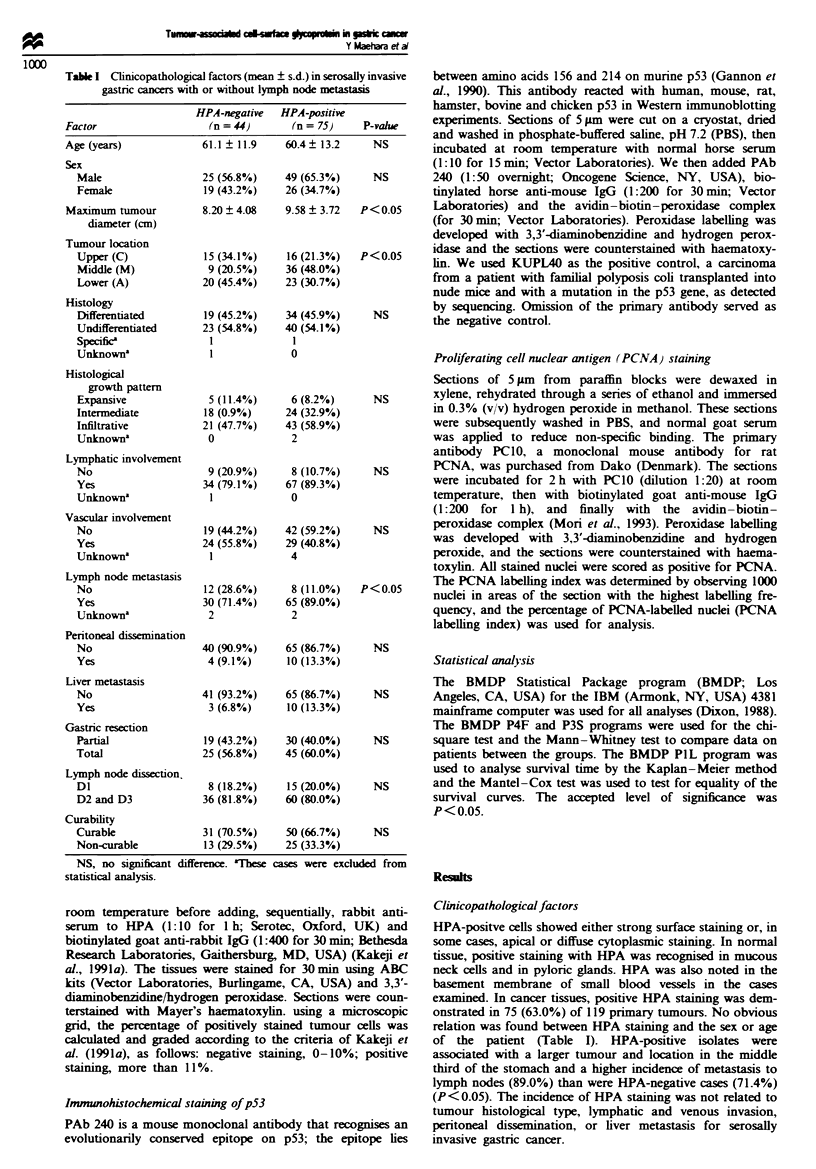
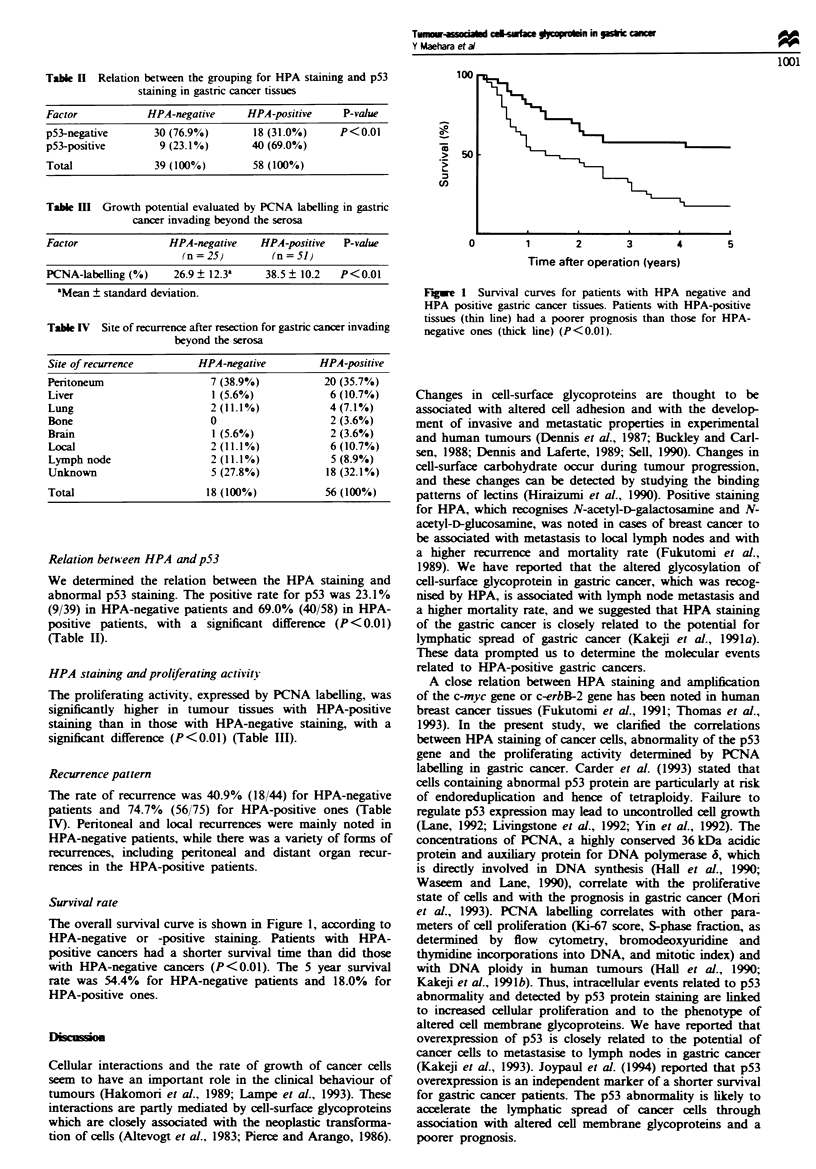
Abstract
Tumour-associated cell-surface glycoprotein is associated with tumour progression in gastric cancer. We investigated the biological significance of tumour-associated cell-surface glycoprotein, determined by the binding of Helix pomatia agglutinin (HPA), with regard to survival time and to the malignant potential of cancer cells in serosally invasive gastric cancer in 119 patients. HPA was positively stained in 75 of 119 patients (63.0%) with gastric cancer with serosal invasion. In patients with HPA-positive tissue, the tumour was larger than in HPA-negative cases and was frequently located in the middle third of the stomach. The incidence of lymph node metastasis was higher than in patients with HPA-negative tissue. There were no differences between the cases staining negatively and positively with HPA with respect to the other factors examined. Gastric cancer tissues with HPA-positive staining revealed a higher positive rate of abnormal p53 staining and a higher concentration of proliferating cell nuclear antigen (PCNA) labelling. The survival time of the patients with HPA positive staining was shorter than for those whose tissues were HPA negative. Thus, tumour-associated cell-surface glycoprotein is apparently closely related to the malignant potential of serosally invasive gastric cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altevogt P., Fogel M., Cheingsong-Popov R., Dennis J., Robinson P., Schirrmacher V. Different patterns of lectin binding and cell surface sialylation detected on related high- and low-metastatic tumor lines. Cancer Res. 1983 Nov;43(11):5138–5144. [PubMed] [Google Scholar]
- Buckley N. D., Carlsen S. A. Involvement of soybean agglutinin binding cells in the lymphatic metastasis of the R3230AC rat mammary adenocarcinoma. Cancer Res. 1988 Mar 15;48(6):1451–1455. [PubMed] [Google Scholar]
- Carder P., Wyllie A. H., Purdie C. A., Morris R. G., White S., Piris J., Bird C. C. Stabilised p53 facilitates aneuploid clonal divergence in colorectal cancer. Oncogene. 1993 May;8(5):1397–1401. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S. Oncodevelopmental expression of--GlcNAc beta 1-6Man alpha 1-6Man beta 1--branched asparagine-linked oligosaccharides in murine tissues and human breast carcinomas. Cancer Res. 1989 Feb 15;49(4):945–950. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Fukutomi T., Hirohashi S., Tsuda H., Nanasawa T., Yamamoto H., Itabashi M., Shimosato Y. The prognostic value of tumor-associated carbohydrate structures correlated with gene amplifications in human breast carcinomas. Jpn J Surg. 1991 Sep;21(5):499–507. doi: 10.1007/BF02470985. [DOI] [PubMed] [Google Scholar]
- Fukutomi T., Itabashi M., Tsugane S., Yamamoto H., Nanasawa T., Hirota T. Prognostic contributions of Helix pomatia and carcinoembryonic antigen staining using histochemical techniques in breast carcinomas. Jpn J Clin Oncol. 1989 Jun;19(2):127–134. [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hiraizumi S., Takasaki S., Shiroki K., Kochibe N., Kobata A. Transfection with fragments of the adenovirus 12 gene induces tumorigenicity-associated alteration of N-linked sugar chains in rat cells. Arch Biochem Biophys. 1990 Jul;280(1):9–19. doi: 10.1016/0003-9861(90)90511-v. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Joypaul B. V., Hopwood D., Newman E. L., Qureshi S., Grant A., Ogston S. A., Lane D. P., Cuschieri A. The prognostic significance of the accumulation of p53 tumour-suppressor gene protein in gastric adenocarcinoma. Br J Cancer. 1994 May;69(5):943–946. doi: 10.1038/bjc.1994.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeji Y., Korenaga D., Tsujitani S., Baba H., Anai H., Maehara Y., Sugimachi K. Gastric cancer with p53 overexpression has high potential for metastasising to lymph nodes. Br J Cancer. 1993 Mar;67(3):589–593. doi: 10.1038/bjc.1993.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeji Y., Korenaga D., Tsujitani S., Haraguchi M., Maehara Y., Sugimachi K. Predictive value of Ki-67 and argyrophilic nucleolar organizer region staining for lymph node metastasis in gastric cancer. Cancer Res. 1991 Jul 1;51(13):3503–3506. [PubMed] [Google Scholar]
- Kakeji Y., Tsujitani S., Mori M., Maehara Y., Sugimachi K. Helix pomatia agglutinin binding activity is a predictor of survival time for patients with gastric carcinoma. Cancer. 1991 Dec 1;68(11):2438–2442. doi: 10.1002/1097-0142(19911201)68:11<2438::aid-cncr2820681119>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kawai T., Suzuki M., Torikata C., Suzuki Y. Expression of blood group-related antigens and Helix pomatia agglutinin in malignant pleural mesothelioma and pulmonary adenocarcinoma. Hum Pathol. 1991 Feb;22(2):118–124. doi: 10.1016/0046-8177(91)90032-k. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Yanoshita R., Konishi M., Ito S., Seki M., Tanaka K., Maeda Y., Iino H., Fukayama M., Koike M., Mori T. Genetic changes of both p53 alleles associated with the conversion from colorectal adenoma to early carcinoma in familial adenomatous polyposis and non-familial adenomatous polyposis patients. Cancer Res. 1992 Jul 15;52(14):3965–3971. [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Maehara Y., Kohnoe S., Sugimachi K. Chemosensitivity test for carcinoma of digestive organs. Semin Surg Oncol. 1990;6(1):42–47. doi: 10.1002/ssu.2980060109. [DOI] [PubMed] [Google Scholar]
- Maehara Y., Sakaguchi Y., Moriguchi S., Orita H., Korenaga D., Kohnoe S., Sugimachi K. Signet ring cell carcinoma of the stomach. Cancer. 1992 Apr 1;69(7):1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::aid-cncr2820690702>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Mori M., Kakeji Y., Adachi Y., Moriguchi S., Maehara Y., Sugimachi K., Jessup J. M., Chen L. B., Steele G. D., Jr The prognostic significance of proliferating cell nuclear antigen in clinical gastric cancer. Surgery. 1993 Jun;113(6):683–690. [PubMed] [Google Scholar]
- Olden K. Adhesion molecules and inhibitors of glycosylation in cancer. Semin Cancer Biol. 1993 Oct;4(5):269–276. [PubMed] [Google Scholar]
- Pierce M., Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986 Aug 15;261(23):10772–10777. [PubMed] [Google Scholar]
- Schumacher U., Kretzschmar H., Brooks S., Leathem A. Helix pomatia lectin binding pattern of brain metastases originating from breast cancers. Pathol Res Pract. 1992 Apr;188(3):284–286. doi: 10.1016/s0344-0338(11)81205-7. [DOI] [PubMed] [Google Scholar]
- Sell S. Cancer-associated carbohydrates identified by monoclonal antibodies. Hum Pathol. 1990 Oct;21(10):1003–1019. doi: 10.1016/0046-8177(90)90250-9. [DOI] [PubMed] [Google Scholar]
- Thomas M., Noguchi M., Fonseca L., Kitagawa H., Kinoshita K., Miyazaki I. Prognostic significance of Helix pomatia lectin and c-erbB-2 oncoprotein in human breast cancer. Br J Cancer. 1993 Sep;68(3):621–626. doi: 10.1038/bjc.1993.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S., Tsuda H., Maruyama K., Kinoshita T., Sasako M., Saito T., Kobayashi M., Hirohashi S. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer. 1993 Dec 1;72(11):3179–3184. doi: 10.1002/1097-0142(19931201)72:11<3179::aid-cncr2820721108>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- von Lampe B., Stallmach A., Riecken E. O. Altered glycosylation of integrin adhesion molecules in colorectal cancer cells and decreased adhesion to the extracellular matrix. Gut. 1993 Jun;34(6):829–836. doi: 10.1136/gut.34.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]



