Abstract
We have used immunoprecipitation with mAbs to probe folding during biosynthesis of the β2 integrin subunit of lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18) before and after association with the αL subunit. An evolutionarily conserved region is present in the β2 subunit between amino acid residues 102 and 344. mAbs to one subregion before the conserved region, and two subregions after the conserved domain, immunoprecipitated both the unassociated β′2 precursor and mature αL/β2 complex, suggesting portions of these subregions are folded before association with αL. An activating mAb to the C-terminal cysteine-rich region, KIM127, preferentially bound to the unassociated β subunit, suggesting that it may bind to an epitope that is in an αβ interface in unactivated LFA-1. By contrast, mAbs to five different epitopes in the conserved region did not react with unassociated β′2 precursor, suggesting that this region folds after αL association and is intimately associated with the αL subunit in the αL/β2 complex. mAbs to two different epitopes that involve the border between the conserved region and the C-terminal segment, were fully or partially reactive with the β′2 precursor, suggesting that this region is partially folded before association with αL. The findings suggest that the conserved region is a distinct folding and hence structural unit, and is intimately associated with the α subunit.
Integrins are a family of cell surface glycoproteins that mediate cell–cell and cell–substrate adhesion, extracellular matrix assembly, and signal transduction (1). Integrins are heterodimers consisting of noncovalently associated α and β subunits. The leukocyte integrins are restricted to leukocytes and share the β2 integrin or CD18 subunit (2, 3). The β2 subunit can form four different αβ complexes containing the αL (CD11a) subunit of lymphocyte function-associated antigen 1 (LFA-1), the αM (CD11b) subunit of Mac-1, the αX (CD11c) subunit of p150,95, and the αD subunit of αD/β2 (4–6). The leukocyte integrins are essential in many phases of immune and inflammatory responses (7). Mutations in the β2 integrin subunit are responsible for leukocyte adhesion deficiency I and result in recurring and life-threatening infections (8, 9).
Much remains to be learned about the structure of integrins. An overall view is provided by electron microscopy, which shows a globular head region that binds ligand, contains the N-terminal portions of the α and β subunits and is connected to the membrane by two stalks corresponding to extended, more C-terminal regions of the α and β subunits (10). Several domains or subregions in integrins have been identified. The N-terminal region of integrin α subunits contains seven repeats of ≈60 aa each that have been predicted to fold into a cylindrical structure with seven β-sheets known as a β-propeller domain (11). An inserted (I) domain is located between β-sheet 2 and β-sheet 3 of the β-propeller domain in 7 of the 16 different integrin α subunits characterized in mammals. The I domain can be expressed as an isolated domain (12). The three-dimensional structure of the I domain shows that it has a nucleotide-binding fold shared with G proteins and a metal ion-dependent adhesion site (13, 14).
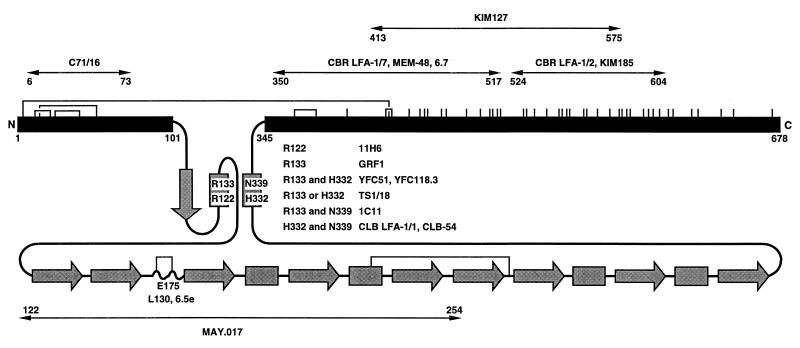
Several subregions are apparent in integrin β subunits. A region of ≈240-aa residues (residues 102–344 in β2) shows the highest conservation between species and among different subunits and has been termed the conserved region or conserved domain, although there is little evidence that it is a structural unit. This region has a metal ion-dependent adhesion site-like site and has been predicted to fold into an I domain-like structure (13, 15). However, the exact boundaries and fold of this region are debatable. A fold identical to an I domain has been proposed for residues in β3 that correspond to residues 102–284 in β2 (15); however, this omits the last 60 residues of the region defined by sequence conservation. We have proposed a modified fold that encompasses the entire conserved region (C.H. and T.A.S., unpublished data). Evidence that the domain boundaries indeed correspond to the conserved region comes from three mAbs that bind combinatorial epitopes involving both Arg-133 and His-332, and one mAb that binds both Arg-133 and Asn-339. These residues are in the first and last predicted α-helices in the conserved region, which are predicted to be adjacent in the structure. It is notable that in the I domain, the first and last α-helices are also adjacent (13, 14). The vast majority of mutations in leukocyte adhesion deficiency I map to the conserved region (see ref. 16). Because these mutations block association of α and β subunits during biosynthesis of integrins, the β subunit conserved domain is important for association with α (8). Site-directed mutagenesis and cross-linking experiments suggest that the conserved region is important in ligand binding (17). Furthermore, both function-blocking and activating mAbs map to this region (18, 19) (C.H. and T.A.S., unpublished data).
A cysteine-rich region is present in the C-terminal portion of the β subunit extracellular domain, from residues 423 to 609. The 20% content of cysteine suggests that this region is highly rigid. Interestingly, a number of mAbs that activate or report conformational changes map to this region in β1, β2, and β3 (20–26) (C.H. and T.A.S., unpublished data).
Here, we study the folding during biosynthesis of the integrin β2 subunit. Previous studies on leukocyte integrin biosynthesis have shown that the α and β subunit precursors are initially unassociated and have high mannose N-linked carbohydrate, and that processing to complex carbohydrate with an accompanying increase in Mr does not occur until after α and β subunit association (4, 8, 27, 28). Thus, transport from the endoplasmic reticulum to the Golgi apparatus is dependent on formation of the αβ complex. In general, the leukocyte integrin β subunit is produced in excess over the α subunits and requires substantially longer to be chased from the precursor to the mature form. We demonstrate by reactivity with mAb that regions of the β2 subunit extracellular domain that precede and follow the conserved domain are folded before association with αL. Folding of the conserved domain is not completed until after association with αL; however, portions of the predicted C-terminal α-helix and possibly the N-terminal α-helix appear to be folded before association with αL.
MATERIALS AND METHODS
mAbs and Cell Lines.
The mouse anti-human CD18 mAbs TS1/18, CBR LFA-1/2, CBR LFA-1/7, CD11a mAb TS1/22, and X63 myeloma IgG1 were described (26, 29). Antibody 1C11 was obtained through the Fourth International Leukocyte Workshop. Antibodies 6.7 (30), CLB LFA-1/1 (31), and L130 were obtained through the Fifth International Leukocyte Workshop. CLB-54 was a gift from R. Van Lier (University of Amsterdam). 11H6 was a gift from H. J. Bühring (Med Klinik II, FACS Laboratory, Tübingen, Germany). Rat antibodies YFC51.1 and YFC118.3 were gifts from G. Hale (32). GRF1 was a gift from F. Garrido (Servicio de Analisis Clinicos, Spain). MEM-48 was a gift from V. Horejsí (Institute of Molecular Genetics, Czechoslovakia). MAY.017 was a gift of Y. Ohashi (33). KIM127 and KIM185 were gifts of M. Robinson (34). Many of these mAbs were characterized in the Fourth (35) and Fifth (36) International Leukocyte Workshops.
The JY B lymphoblastoid line, and the mutant Jurkat-β2.7 T lymphoblastoid cell line that lacks the LFA-1 α subunit but retains the β2 subunit (37), were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and 50 μg/ml gentamicin. The murine T lymphoblastoid cell line EL-4 was grown in the same medium containing an additional 50 μM 2-mercaptoethanol.
Metabolic Labeling, Immunoprecipitation, and Electrophoresis.
Cultured cells were washed once with cysteine-free RPMI 1640 medium plus 15% dialyzed fetal bovine serum and were resuspended to 5 × 106 cells/ml in the same medium. After incubation for 45 min, cells were pulsed with 500 μCi/ml (1 Ci = 37 GBq) [35S]cysteine (ICN) for 1 h. Half of the cells were harvested and the remaining cells were chased for 12–16 h by adding an equal volume of RPMI 1640 medium containing 500 μg/ml cysteine. Labeled cells were lysed with Triton X-100 buffer, and lysates were subjected to immunoprecipitation, reducing SDS/PAGE, and fluorography as described (38).
RESULTS
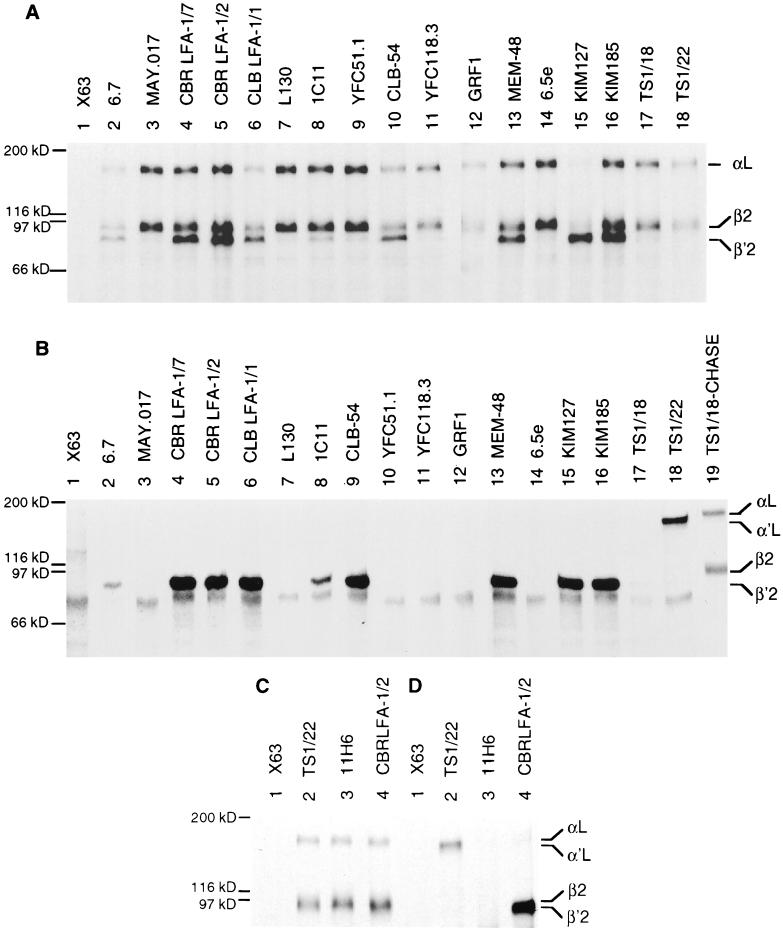
A panel of mouse and rat mAbs specific for the human β2 integrin subunit, and one rat mAb specific for the mouse β2 integrin subunit, were used in this study. Eleven mAb recognize 7 different epitopes in the conserved region, all but 1 of which are defined at the level of individual human/mouse amino acid substitutions (C.H. and T.A.S., unpublished data) (Fig. 1). Seven mAbs recognize epitopes in the subregion preceding the conserved domain and in at least two subregions following the conserved domain. All mAbs to human β2 could immunoprecipitate the human LFA-1 αL/β2 complex from lysates of JY cells pulsed for 1 h with [35S]cysteine and chased for 16 h, as shown by coprecipitation of the mature αL and β2 subunits (Fig. 2A, lanes 2–17; Fig. 2C, lanes 3, 4). However, mAb KIM127 precipitated very little mature αL and β2 (Fig. 2A, lane 15). The mature αL and β2 subunits were also precipitated by a mAb to the αL subunit (Fig. 2A, lane 18). Although the absolute amount of mature αL and β2 precipitated by different mAbs varied, the ratio of the intensities of the αL and β2 subunits was the same in all cases, with slightly more label in β2 than αL.
Figure 1.
Schematic diagram of the extracellular domain of the integrin β2 subunit. The conserved domain, from residues 102 to 344, is shaded gray and shown schematically according to its predicted secondary structure (C.H. and T.A.S., unpublished data). Arrows and rectangles represent predicted β-strands and α-helices, respectively. The regions from amino acids 1–101 and 345 to the end of the extracellular domain at 678 are shown as black bars and are to scale with one another but not with the conserved domain. All cysteines are shown as vertical lines, and the best characterized disulfides in β3 (39) are shown as connections between the homologues cysteines in β2. The localization of species-specific residues recognized by mAbs is described elsewhere (C.H. and T.A.S., unpublished data). Regions were mapped with mouse-human β2 chimeras and single amino acid substitutions. The regions defined by chimeras are further delimited by excluding regions at their borders identical between mouse and human, and thus regions specified in the figure are narrower than reported elsewhere.
Figure 2.
Immunoprecipitation of LFA-1 subunits from JY lymphoblastoid cells. (A and C) Cells were pulsed for 1 h with [35S]cysteine and chased for 16 h with unlabeled cysteine. (B and D) Cells were pulsed for 1 h with [35S]cysteine. (A and B) Lysates were immunoprecipitated with X63 myeloma IgG1 as control (lane 1), mouse anti-human β subunit (lanes 2–17), and mouse anti-human αL subunit (lane 18) mAbs. As a control, mature LFA-1 was precipitated with TS1/18 mAb from lysates of JY cells that were pulsed and chased for 16 h (B, lane 19). (C and D) A separate experiment with 11H6 mAb (lane 3), a batch of which was obtained after other work was completed. Controls with X63 myeloma IgG1 (lane 1), mAb to αL (lane 2), and CBR LFA-1/2 mAb to β2 (lane 4) were included. Precipitates were subjected to reducing SDS/7.5% PAGE and fluorography. The positions of mature αL, α′L precursor, mature β2, and β′2 precursor subunits are indicated. Molecular weight standards are myosin (200 kDa), β-galactosidase (116 kDa), phosphorylase b (97 kDa), and serum albumin (66 kDa).
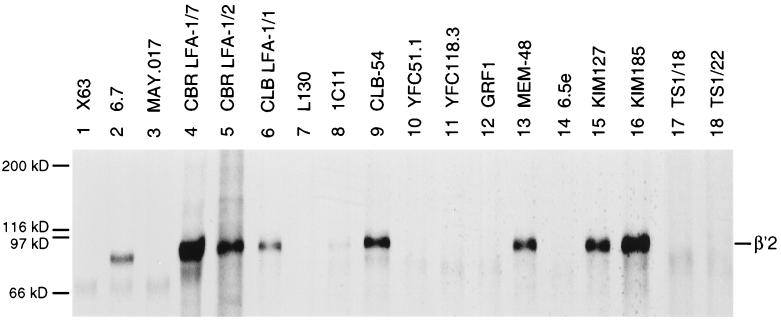
A subset of mAbs precipitated the β′2 precursor in addition to mature αL and β2. These include mAbs that bind to epitopes in the two subregions C terminal to the conserved domain (Fig. 2A, lanes 2, 4, 5, 13, 15, and 16). Even among mAbs that precipitated the mature αLβ2 complex weakly, some detectably precipitated the β′2 precursor (Fig. 2A, lanes 2, 6, 8, and 15). Interestingly, KIM127, which is an activating mAb that binds to cells at 37°C but not at 4°C and maps to the cysteine rich region (21, 40), preferentially precipitated the β′2 precursor compared with mature αL/β2 (Fig. 2A, lane 15). Two mAbs that bind a combinatorial epitope involving H332 and N339 predicted to lie in the last half of the C-terminal α-helix of the conserved domain also precipitated the β′2 precursor (Fig. 2A, lanes 6 and 10). Moreover, one mAb that binds a combinatorial epitope involving R133 and N339, which are predicted to lie in the first and last α-helices, respectively, weakly precipitated the β′2 precursor (Fig. 2A, lane 8). By contrast, eight mAbs that map to four other epitopes in the conserved domain precipitated little if any β′2 precursor (Fig. 2A, lanes 3, 7, 9, 11, 12, 14, and 17; Fig. 2C, lane 3).
Association of β′2 with α′L precedes carbohydrate processing, resulting in the maturation to β2 and αL; therefore, even mAbs specific for the α subunit can precipitate small amounts of β′2 (27). To better study reactivity with unassociated β′2, cells were pulse labeled for 1 h. Under these conditions, mAbs to β2 precipitated only β′2 precursor (Fig. 2B, lanes 2, 4–6, 8, 9, 13, 15, and 16), and mAbs to αL precipitated only α′L precursor (Fig. 2B, lane 18). Significant αβ association does not occur until later time points, as shown previously (4, 41) and confirmed after a 16-h chase (Fig. 2B, lane 19). The same reactivity with β′2 precursor was seen as with chased cells. mAbs to subregions C-terminal to the conserved domain precipitated β′2 (Fig. 2B, lanes 2, 4, 5, 13, 15, and 16), as did mAb to the epitope involving H332 and N339 in the conserved domain (Fig. 2B, lanes 6 and 9). Again, precipitation by mAb 1C11 to the epitope involving R133 and N339 was weak (Fig. 2B, lane 8). Furthermore, mAbs to the five other epitopes in the conserved domain did not precipitate the β′2 precursor (Fig. 2B, lanes 3, 7, 10–12, 14, and 17; Fig. 2D, lane 3).
To further discriminate between β2 subunit epitopes that were dependent and independent of subunit association, we utilized a mutant Jurkat T lymphoblastoid cell line that synthesizes β′2 but not α′L precursor and lacks surface expression of LFA-1 (37). Cells were pulsed with [35S]cysteine for 1 h and chased for 12 h to allow a long time for β2 subunit folding. The same reactivities with the β′2 precursor were observed as in pulsed and pulse-chased JY cells. mAbs to subregions following the conserved domain (Fig. 3, lanes 2, 4, 5, 13, 15, and 16) and to H332 and N339 (Fig. 3, lanes 6 and 9) precipitated β′2 precursor; the mAbs to R133 and N339 precipitated β′2 precursor weakly (Fig. 3, lane 8); and mAbs to the four other conserved domain epitopes did not precipitate β′2 (Fig. 3, lanes 3, 7, 10–12, 14, and 17). As shown previously (37), nothing was precipitated with a mAb to αL (Fig. 3, lane 18).
Figure 3.
Immunoprecipitation of β′2 subunit precursor from mutant Jurkat-β2.7 cells that lack leukocyte integrin α subunits. Mutant Jurkat-β2.7 T lymphoblastoid cells were pulsed with [35S]cysteine for 1 h and chased with unlabeled cysteine for 12 h. Lysates were immunoprecipitated with X63 myeloma IgG1 control (lane 1), mouse anti-human LFA-1 β subunit (lanes 2–17), and mouse anti-human LFA-1 α subunit (lane 18) mAbs and subjected to reducing SDS/7.5% PAGE. The position of the β′2 precursor is indicated.
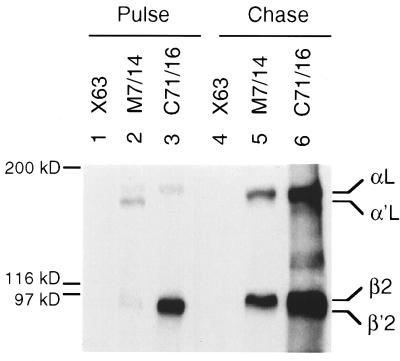
Folding of the region N-terminal to the conserved domain was studied with the rat C71/16 mAb to murine β2. After a 1-h pulse little β′2 or β2 were associated with αL and α′L as shown by precipitation with anti-αL mAb (Fig. 4, lane 2), yet a large amount of β′2 precursor was precipitated by the mAb to β2 (Fig. 4, lane 3). After a 16-h chase, the mAb to αL precipitated only mature αL and β2, whereas the mAb to β2 precipitated αL, β2, and β′2, consistent with an excess of β′2 over α′L and the longer time required for maturation of β′2 (4, 27, 41). These findings suggest that the C71/16 mAb reacts with unassociated β′2, and that its epitope in the region N-terminal to the conserved domain is folded before association with α′L.
Figure 4.
Immunoprecipitation of pulse-labeled and pulse-chased murine LFA-1. The murine T cell line, EL-4 was pulsed for 1 h with [35S]cysteine, and half of the cells were chased for 16 h with unlabeled cysteine. Cell lysates were immunoprecipitated with control X63 myeloma IgG1, rat anti-mouse LFA-1 α subunit mAb M7/14, or rat anti-mouse β2 mAb C71/16. The precipitates were subjected to reducing SDS/7.5% PAGE. The positions of the mature αL, precursor α′L, mature β2, and precursor β′2 subunits are indicated.
DISCUSSION
We have used mAbs to probe the folding during biosynthesis of the integrin β2 subunit. We have demonstrated that folding of N-terminal and C-terminal segments of the β2 subunit is independent of the conserved domain, and that folding of most of the conserved domain requires association with α. We believe it is reasonable to suppose that the mAbs studied here, like all other characterized mAbs, are conformation-dependent (42) and thus good probes of folding. Furthermore, a number of the mAbs studied here have been tested in Western blot analyses and have been found to react with nonreduced but not reduced β2, demonstrating dependence on conformation (21, 26).
Regions N-terminal and C-terminal to the conserved domain were found to be folded before α subunit association. One epitope was present on the unassociated β subunit precursor in the region between residues 6 and 73. Epitopes defined by three different mAbs were present on the unassociated β subunit precursor between residues 350 and 517, and epitopes recognized by two mAbs were present between residues 524 and 604 (Fig. 1). It is not known whether mAbs that map to the same subregion recognize the same or different human/mouse amino acid substitutions, but species-specific differences in these latter two regions are abundant. Another mAb, KIM127, was mapped in a separate study to residues 413–575 (21). KIM127 and KIM185 recognize spatially distinct epitopes because they do not cross-block one another; furthermore, although CBR LFA-1/2 and KIM127 are both activating mAbs, the epitope for KIM127 is temperature-dependent (40) whereas that of CBR LFA-1/2 is not (26). Because mAbs to protein antigens generally interact over a surface area of 750–900 Å (43), our results suggest that substantial portions of the regions N-terminal and C-terminal to the conserved region adopt a near-native protein fold before association with leukocyte integrin α subunits.
There are several interesting correlations between our results and work on the disulfide bond structure and protease-susceptibility of the integrin β3 subunit (39, 44). All 56 cysteines in integrin β2 and β3 are conserved and the disulfide bond structure is expected to be identical (39). Both of the folded regions defined here are rich in disulfides (Fig. 1). Furthermore, there is a long-range disulfide bond between these two regions that links residues Cys-5 and Cys-435 (39). Approximately the same regions are highly resistant to digestion under native conditions of αIIb/β3 with a variety of proteases and remain in a “core” that contains residues 1–52 and 423–622 (44). Therefore, the regions N-terminal and C-terminal of the conserved domain in the β2 subunit not only appear folded before association with α, but may well fold together with one another into a common structural unit.
By contrast, substantial portions of the conserved domain appear not to fold until after association with α. Nine mAb to five different epitopes within the conserved domain did not precipitate the unassociated β subunit, but precipitated the αβ complex. These mAbs do not require species-specific residues from the α subunit because they react equally well with human αL/human β2 and murine αL/human β2 as demonstrated by immunofluorescence with mouse × human hybrid T cell lines (45) (data not shown). A caveat is that non-species-specific portions of the α subunit could be required for completion of a species-specific β subunit epitope. However, because species-specific substitutions are expected to be present near the center of epitopes, this interpretation appears less likely than dependence on association for folding, particularly as a general explanation for our results with multiple mAbs to five different epitopes. The mAbs that recognized β only after association with α include three specific for residue E175, one specific for residue R122, one specific for residue R133, and three specific for a combinatorial epitope involving R133 and H332 (C.H. and T.A.S., unpublished data). Within this latter group specificities differ, since reactivity with mAb TS1/18 is lost only when both R133 and H332 are substituted with corresponding mouse residues, whereas reactivity with YFC51.1 and YFC118.3 is lost when either residue is substituted. A further mAb, MAY.017, could not be assigned to a specific amino acid residue, but appears to recognize a combinatorial epitope involving residues both in the region of 122–163 and 164–254. The results with these mAbs suggest that a substantial portion of the conserved domain is at least partially unfolded in the unassociated β2 subunit, and that folding is not completed until after association with the α subunit. Differential reactivity with αβ compared with free β has also been observed using a smaller set of β2 mAb with secreted, truncated αx/β2 (46) or αL/β2 (C.L. and T.A.S., unpublished data) compared with secreted, truncated β2.
It was interesting that three mAbs to two different epitopes in the conserved domain could recognize the unassociated β subunit, and that all recognized as part of their epitope N339, which is on the border of this domain. Two mAbs recognize H332 and N339, which are predicted to be on the same face of an α-helix that is the final secondary structure element in one model of the conserved domain (C.H. and T.A.S., unpublished data). Reactivity of these mAbs is lost when N339 is substituted, and only partially lost when H332 is substituted. The ratio of αL/β2 complex to β′2 precursor precipitated by these two mAbs appeared to be the same as for mAbs to the C-terminal regions that are independent of association (Fig. 2), which suggests that this putative α-helix, or at least the part of the helix containing residue N339, and surrounding regions recognized by these mAbs, are almost fully folded before association with αL. The 1C11 mAb recognizes N339 and R133. The latter residue is predicted to be on the first α-helix in the conserved domain; helices bearing R133 and N339 may correspond to the neighboring α-helices 1 and 7 in the I domain. Reactivity with 1C11 mAb is lost when both R133 and N339 are substituted with mouse residues and retained when only one is substituted. The 1C11 mAb clearly reacts with the unassociated β2 precursor, but less well than with the αL/β2 complex. This could suggest that there is some association between these two α-helices before association with the α subunit. The conserved domain might be partially folded in the unassociated β subunit, or folding together of the N-terminal and C-terminal segments of the β subunit might bring these regions together. However, because reactivity is retained when either R133 or N339 is substituted, an alternative possibility is that antibody binding could occur even without association between the two predicted α-helices.
Our results on the conserved domain of β2 correlate with proteolysis and disulfide studies on this region in β3. In native αIIb/β3, the regions between residues 100–150 and 308–324 are the most sensitive to proteolysis (44), and the conserved domain has only two disulfide bonds (Fig. 1) (39).
We report elsewhere that in the LFA-1 αL subunit, subregions in the β-propeller domain require β subunit association for folding, whereas folding of the I domain is independent of the β-propeller domain and association with the β subunit (38). Probes were unavailable to assess the status of a substantial portion of the α subunit extracellular domain that is C-terminal to these two domains. Since the N-terminal regions of the integrin α and β subunits appear to associate as shown by electron microscopy (10), these results may suggest that the β subunit conserved domain and the α subunit β-propeller domain associate with one another; however, other regions that have not been probed with mAbs might also be unfolded in the isolated α and β subunits and contribute to the observed interactions. Because both the β-propeller domain and the conserved domain are important in ligand binding, the mode of interaction between them is of considerable interest. The Ca2+-binding motifs in integrin α subunits are predicted to be in close proximity to one another on the lower surface of the β-propeller domain (11). Because removal of Ca2+ weakens association between integrin α and β subunits (47, 48), and can activate ligand binding by LFA-1 (49), this surface may be involved in interaction with the β subunit, and in particular with the conserved domain.
Our studies suggest that among several subregions of the β subunit, the conformation of the conserved domain is most dependent on α subunit association. However, other regions may also participate in subunit interfaces, while retaining conformations more nearly resembling those present in the unassociated α and β subunits. This may be the case in the cysteine-rich, C-terminal portion of the β subunit. A number of mAbs that can induce integrin activation or report activation map to C-terminal regions of the β subunits (20, 22–26) (C.H. and T.A.S., unpublished data). KIM127 mAb can induce integrin activation (40) and bound preferentially to the unassociated β2 subunit compared with the αβ complex. This mAb, binding of which to β2 integrins on the cell surface is temperature-dependent (40), may bind to a region of the β2 subunit that is buried in an interface with α in unactivated integrins and is exposed in an alteration in the αβ interface that is associated with activation.
In conclusion, our results show that folding of the conserved domain is not completed until after association with the integrin αL subunit. By contrast, no dependence on association with αL was found for the folding of the region N-terminal to the conserved domain, and two subregions C-terminal to the conserved domain. Our results have implications for the domain structure of β2 and provide support for the idea that the conserved region of the integrin β subunit is a domain and is intimately associated with the integrin α subunit. Together with previous studies, this information contributes to a low resolution model of tertiary and quaternary associations within integrin αβ complexes and may be useful in guiding efforts aimed at higher resolution structural studies.
Acknowledgments
We thank many colleagues mentioned in Materials and Methods who contributed mAbs for this study. This work was supported by National Institutes of Health Grant CA31798.
ABBREVIATION
- LFA-1
lymphocyte function-associated antigen 1
References
- 1.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T K, O’Connor K, Lee A, Roberts T M, Springer T A. Cell. 1987;48:681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- 3.Law S K A, Gagnon J, Hildreth J E K, Wells C E, Willis A C, Wong A J. EMBO J. 1987;6:915–919. doi: 10.1002/j.1460-2075.1987.tb04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Madrid F, Nagy J, Robbins E, Simon P, Springer T A. J Exp Med. 1983;158:1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson R S, Springer T A. Immunol Rev. 1990;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Van der Vieren M, Le Trong H, Wood C L, Moore P F, St. John T, Staunton D E, Gallatin W M. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 7.Springer T A. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto T K, Hollander N, Roberts T M, Anderson D C, Springer T A. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 9.Anderson D C, Springer T A. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 10.Weisel J W, Nagaswami C, Vilaire G, Bennett J S. J Biol Chem. 1992;267:16637–16643. [PubMed] [Google Scholar]
- 11.Springer T A. Proc Natl Acad Sci USA. 1996;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michishita M, Videm V, Arnaout M A. Cell. 1993;72:857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- 13.Lee J-O, Rieu P, Arnaout M A, Liddington R. Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 14.Qu A, Leahy D J. Proc Natl Acad Sci USA. 1995;92:10277–10281. doi: 10.1073/pnas.92.22.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tozer E C, Liddington R C, Sutcliffe M J, Smeeton A H, Loftus J C. J Biol Chem. 1996;271:21978–21984. doi: 10.1074/jbc.271.36.21978. [DOI] [PubMed] [Google Scholar]
- 16.Bilsland C A G, Springer T A. J Leukocyte Biol. 1994;55:501–506. doi: 10.1002/jlb.55.4.501. [DOI] [PubMed] [Google Scholar]
- 17.Loftus J C, Smith J W, Ginsberg M H. J Biol Chem. 1994;269:25235–25238. [PubMed] [Google Scholar]
- 18.Shih D-T, Edelman J M, Horwitz A F, Grunwald G B, Buck C A. J Cell Biol. 1993;122:1361–1371. doi: 10.1083/jcb.122.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada Y, Puzon W. J Biol Chem. 1993;268:17597–17601. [PubMed] [Google Scholar]
- 20.Du X, Gu M, Weisel J W, Nagaswami C, Bennett J S, Bowditch R, Ginsberg M H. J Biol Chem. 1993;268:23087–23092. [PubMed] [Google Scholar]
- 21.Stephens P, Romer J T, Spitali M, Shock A, Ortlepp S, Figdor C, Robinson M K. Cell Adhes Commun. 1995;3:375–384. doi: 10.3109/15419069509081292. [DOI] [PubMed] [Google Scholar]
- 22.Bazzoni G, Shih D-T, Buck C A, Hemler M A. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 23.Yednock T A, Cannon C, Vandevert C, Goldbach E G, Shaw G, Ellis D K, Liaw C, Fritz L C, Tanner L I. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- 24.Puzon-McLaughlin W, Yednock T A, Takada Y. J Biol Chem. 1996;271:16580–16585. doi: 10.1074/jbc.271.28.16580. [DOI] [PubMed] [Google Scholar]
- 25.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 26.Petruzzelli L, Maduzia L, Springer T. J Immunol. 1995;155:854–866. [PubMed] [Google Scholar]
- 27.Ho M-K, Springer T A. J Biol Chem. 1983;258:2766–2769. [PubMed] [Google Scholar]
- 28.Springer T A, Thompson W S, Miller L J, Schmalstieg F C, Anderson D C. J Exp Med. 1984;160:1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Madrid F, Krensky A M, Ware C F, Robbins E, Strominger J L, Burakoff S J, Springer T A. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David V, Leca G, Corvaia N, Le Deist F, Boumsell L, Bensussan A. Cell Immunol. 1991;136:519–524. doi: 10.1016/0008-8749(91)90372-i. [DOI] [PubMed] [Google Scholar]
- 31.Miedema F, Tetteroo P A T, Hesselink W G, Werner G, Spits H, Melief C J M. Eur J Immunol. 1984;14:518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- 32.Pope I, Hale G, Waldmann H. In: Leukocyte Typing IV: White Cell Differentiation Antigens. Knapp W, Dorken B, Gilks W R, Rieber E P, Schmidt R E, Stein H, von dem Borne A E G, Kr, editors. Oxford: Oxford Univ. Press; 1989. pp. 559–560. [Google Scholar]
- 33.Ohashi Y, Tsuchiya S, Fujie H, Minegishi M, Konno T. Tohoku J Exp Med. 1992;167:297–299. doi: 10.1620/tjem.167.297. [DOI] [PubMed] [Google Scholar]
- 34.Andrew D, Shock A, Ball E, Ortlepp S, Bell J, Robinson M. Eur J Immunol. 1993;23:2217–2222. doi: 10.1002/eji.1830230925. [DOI] [PubMed] [Google Scholar]
- 35.Uciechowski P, Schmidt R. In: Leucocyte Typing IV: White Cell Differentiation Antigens. Knapp W, Dorken B, Gilks W R, Rieber E P, Schmidt R E, Stein H, von dem Borne A E G Jr, editors. Oxford: Oxford Univ. Press; 1989. pp. 543–551. [Google Scholar]
- 36.Petruzzelli L, Luk J, Springer T A. In: Leucocyte Typing V: White Cell Differentiation Antigens. Schlossman S F, Boumsell L, Gilks W, Harlan J, Kishimoto T, Morimoto T, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T, Todd R, editors. New York: Oxford Univ. Press; 1995. pp. 1581–1585. [Google Scholar]
- 37.Weber K S C, York M R, Springer T A, Klickstein L B. J Immunol. 1997;158:273–279. [PubMed] [Google Scholar]
- 38.Huang C, Springer T A. Proc Natl Acad Sci USA. 1997;94:3162–3167. doi: 10.1073/pnas.94.7.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calvete J J, Henschen A, González-Rodríguez J. Biochem J. 1991;274:63–71. doi: 10.1042/bj2740063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson M K, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher E C. J Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- 41.Miller L J, Springer T A. J Immunol. 1987;139:842–847. [PubMed] [Google Scholar]
- 42.Laver W G, Air G M, Webster R G, Smith-Gill S J. Cell. 1990;61:553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- 43.Davies D R, Padlan E A, Sheriff S. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- 44.Calvete J J, Mann K, Alvarez M V, López M M, González-Rodríguez J. Biochem J. 1992;282:523–532. doi: 10.1042/bj2820523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marlin S D, Morton C C, Anderson D C, Springer T A. J Exp Med. 1986;164:855–867. doi: 10.1084/jem.164.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luk J M, Springer T A. FASEB J. 1996;10:A1226. (abstr.). [Google Scholar]
- 47.Jennings L K, Phillips D R. J Biol Chem. 1982;257:10458–10466. [PubMed] [Google Scholar]
- 48.Dustin M L, Carpen O, Springer T A. J Immunol. 1992;148:2654–2663. [PubMed] [Google Scholar]
- 49.Dransfield I, Cabañas C, Craig A, Hogg N. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]