Abstract
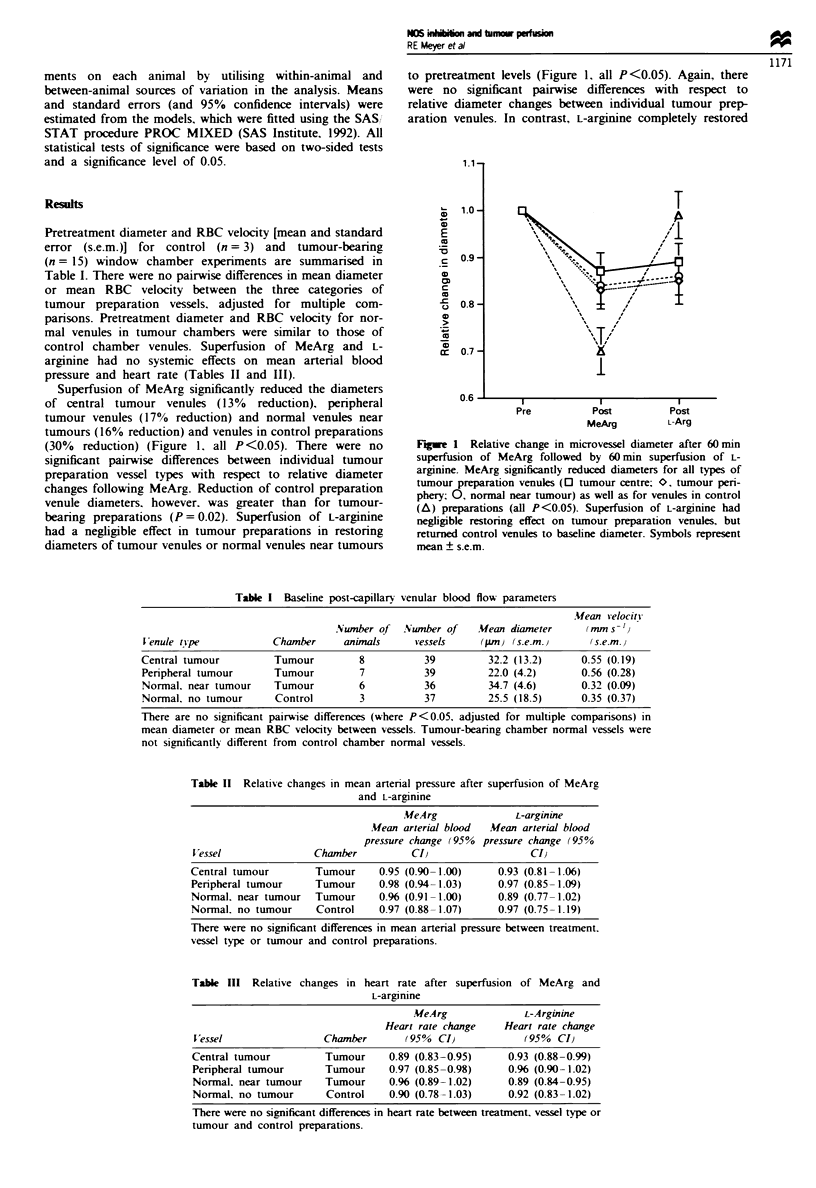
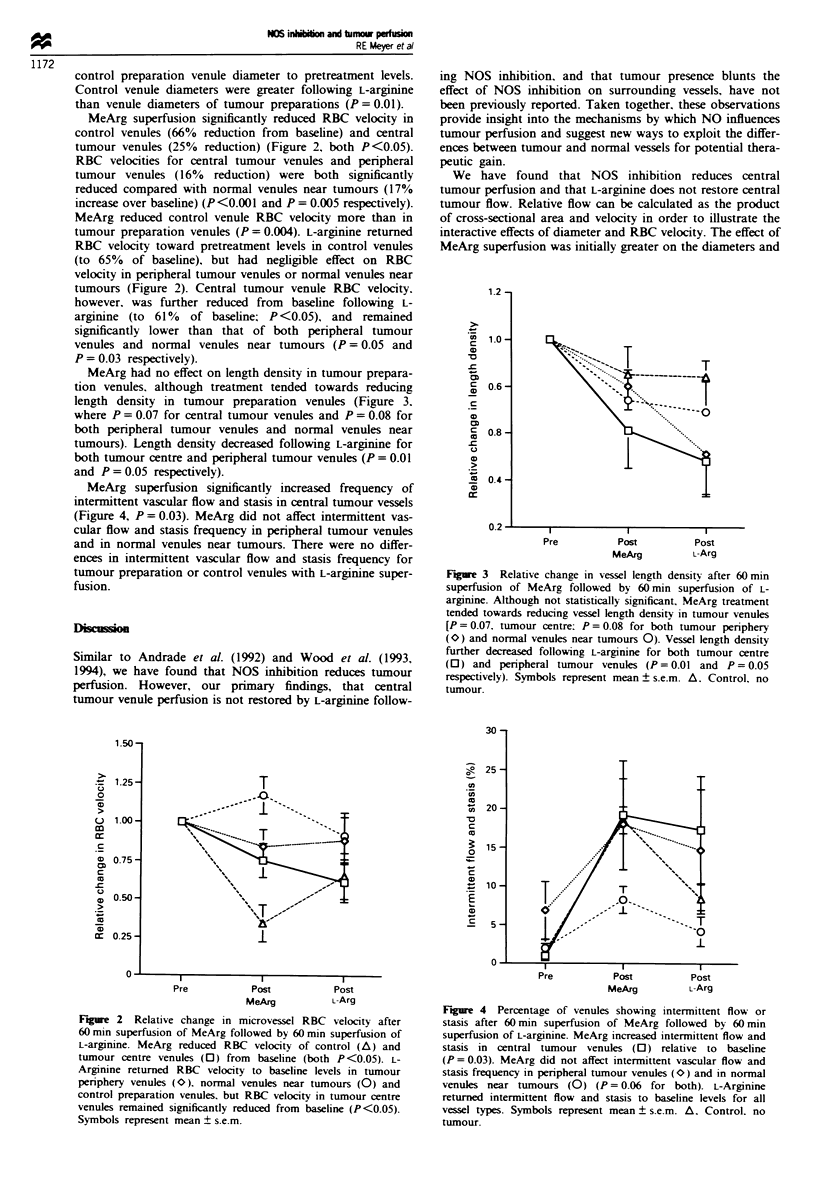
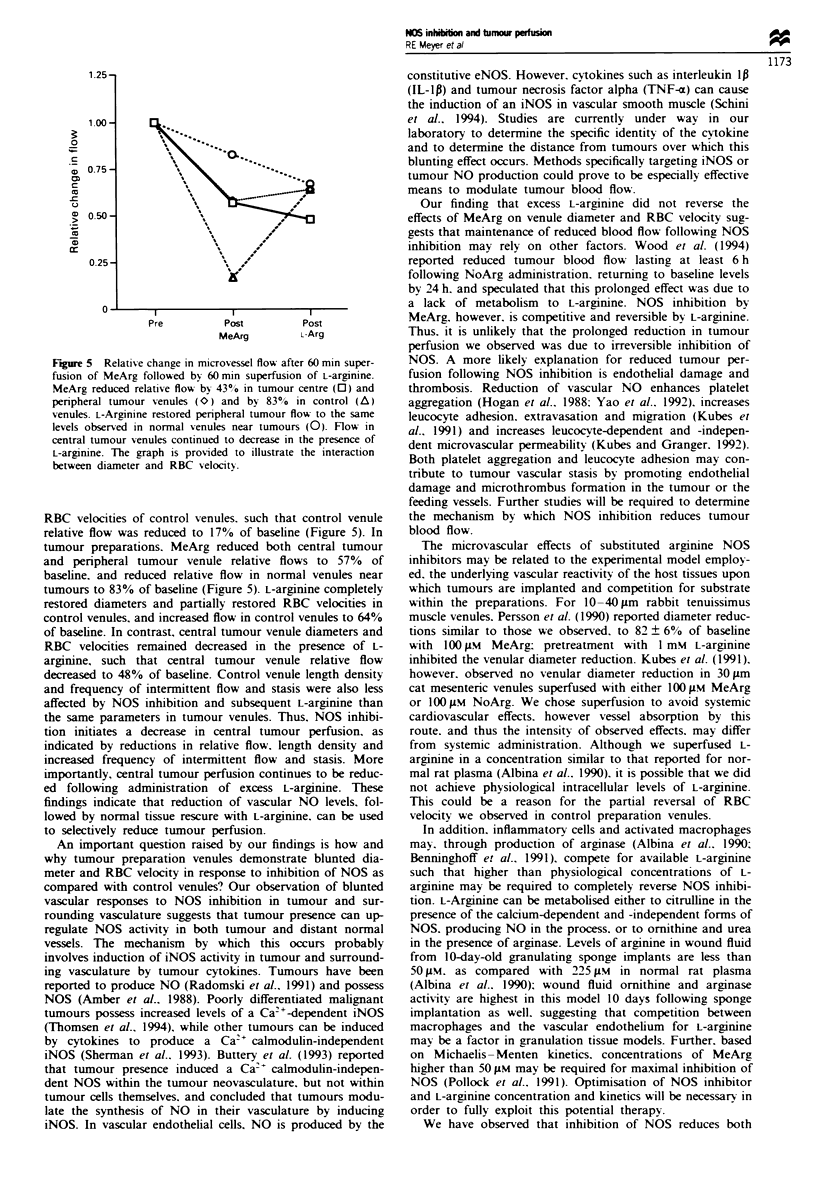
We examined the microvascular effects of competitive nitric oxide synthase (NOS) inhibition with NG-monomethyl-L-arginine (MeArg), followed by L-arginine, on R3230Ac mammary adenocarcinoma perfusion. In window preparations containing tumours, superfusion of 50 microM MeArg reduced diameters of central tumour venules by 13%, of peripheral tumour venules by 17% and of normal venules near tumours by 16% from baseline. MeArg reduced red blood cell (RBC) velocity in central tumour venules by 25%, and increased intermittent flow and stasis frequency by 20% in central tumour venules. Subsequent superfusion of 200 microM L-arginine did not restore diameters or RBC velocity of any tumour preparation venules, and decreased length density in both central tumour venules and peripheral tumour venules. In contrast, MeArg reduced control preparation venule diameter by 30% and RBC velocity by 66%, but did not decrease length density or increase intermittent flow or stasis frequency. Unlike tumour preparation venules, L-arginine restored control venule diameters and velocities. NOS inhibition reduces both tumour and control venule perfusion, but the effect is blunted in the vicinity of tumours, possibly because of increased NOS levels. Perfusion can be subsequently restored in control, but not tumour, venules with L-arginine. Tumour NOS inhibition, followed by normal tissue rescue with L-arginine, may provide a novel means to achieve the goal of selective tumour hypoxia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol. 1990 May 15;144(10):3877–3880. [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. The L-arginine dependent effector mechanism is induced in murine adenocarcinoma cells by culture supernatant from cytotoxic activated macrophages. J Leukoc Biol. 1988 Feb;43(2):187–192. doi: 10.1002/jlb.43.2.187. [DOI] [PubMed] [Google Scholar]
- Andrade S. P., Hart I. R., Piper P. J. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992 Dec;107(4):1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Wayland H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc Res. 1974 Jan;7(1):131–143. doi: 10.1016/0026-2862(74)90043-0. [DOI] [PubMed] [Google Scholar]
- Benninghoff B., Lehmann V., Eck H. P., Dröge W. Production of citrulline and ornithine by interferon-gamma treated macrophages. Int Immunol. 1991 May;3(5):413–417. doi: 10.1093/intimm/3.5.413. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Koong A. Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J Natl Cancer Inst. 1991 Feb 6;83(3):178–185. doi: 10.1093/jnci/83.3.178. [DOI] [PubMed] [Google Scholar]
- Buttery L. D., Springall D. R., Andrade S. P., Riveros-Moreno V., Hart I., Piper P. J., Polak J. M. Induction of nitric oxide synthase in the neo-vasculature of experimental tumours in mice. J Pathol. 1993 Dec;171(4):311–319. doi: 10.1002/path.1711710412. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J. Hydralazine-induced tumor hypoxia: a potential target for cancer chemotherapy. J Natl Cancer Inst. 1989 Apr 19;81(8):618–622. doi: 10.1093/jnci/81.8.618. [DOI] [PubMed] [Google Scholar]
- Chen I. I., Prewitt R. L., Dowell R. F. Microvascular rarefaction in spontaneously hypertensive rat cremaster muscle. Am J Physiol. 1981 Sep;241(3):H306–H310. doi: 10.1152/ajpheart.1981.241.3.H306. [DOI] [PubMed] [Google Scholar]
- Dewhirst M. W., Prescott D. M., Clegg S., Samulski T. V., Page R. L., Thrall D. E., Leopold K., Rosner G., Acker J. C., Oleson J. R. The use of hydralazine to manipulate tumour temperatures during hyperthermia. Int J Hyperthermia. 1990 Nov-Dec;6(6):971–983. doi: 10.3109/02656739009140980. [DOI] [PubMed] [Google Scholar]
- Dewhirst M. W., Secomb T. W., Ong E. T., Hsu R., Gross J. F. Determination of local oxygen consumption rates in tumors. Cancer Res. 1994 Jul 1;54(13):3333–3336. [PubMed] [Google Scholar]
- HILF R., MICHEL I., BELL C., FREEMAN J. J., BORMAN A. BIOCHEMICAL AND MORPHOLOGIC PROPERTIES OF A NEW LACTATING MAMMARY TUMOR LINE IN THE RAT. Cancer Res. 1965 Apr;25:286–299. [PubMed] [Google Scholar]
- Hogan J. C., Lewis M. J., Henderson A. H. In vivo EDRF activity influences platelet function. Br J Pharmacol. 1988 Aug;94(4):1020–1022. doi: 10.1111/j.1476-5381.1988.tb11616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Papenfuss H. D., Gross J. F., Intaglietta M., Treese F. A. A transparent access chamber for the rat dorsal skin fold. Microvasc Res. 1979 Nov;18(3):311–318. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Peterson H. I. Modification of tumour blood flow--a review. Int J Radiat Biol. 1991 Jul-Aug;60(1-2):201–210. doi: 10.1080/09553009114551851. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Jenkins D. C., Holmes L., Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991 Nov 15;51(22):6073–6078. [PubMed] [Google Scholar]
- Schini V. B., Busse R., Vanhoutte P. M. Inducible nitric oxide synthase in vascular smooth muscle. Arzneimittelforschung. 1994 Mar;44(3A):432–435. [PubMed] [Google Scholar]
- Secomb T. W., Hsu R., Dewhirst M. W., Klitzman B., Gross J. F. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys. 1993 Feb 15;25(3):481–489. doi: 10.1016/0360-3016(93)90070-c. [DOI] [PubMed] [Google Scholar]
- Sherman P. A., Laubach V. E., Reep B. R., Wood E. R. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993 Nov 2;32(43):11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- Thomsen L. L., Lawton F. G., Knowles R. G., Beesley J. E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994 Mar 1;54(5):1352–1354. [PubMed] [Google Scholar]
- Wood P. J., Sansom J. M., Butler S. A., Stratford I. J., Cole S. M., Szabo C., Thiemermann C., Adams G. E. Induction of hypoxia in experimental murine tumors by the nitric oxide synthase inhibitor, NG-nitro-L-arginine. Cancer Res. 1994 Dec 15;54(24):6458–6463. [PubMed] [Google Scholar]
- Wood P. J., Stratford I. J., Adams G. E., Szabo C., Thiemermann C., Vane J. R. Modification of energy metabolism and radiation response of a murine tumour by changes in nitric oxide availability. Biochem Biophys Res Commun. 1993 Apr 30;192(2):505–510. doi: 10.1006/bbrc.1993.1444. [DOI] [PubMed] [Google Scholar]
- Yao S. K., Ober J. C., Krishnaswami A., Ferguson J. J., Anderson H. V., Golino P., Buja L. M., Willerson J. T. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation. 1992 Oct;86(4):1302–1309. doi: 10.1161/01.cir.86.4.1302. [DOI] [PubMed] [Google Scholar]


