Abstract
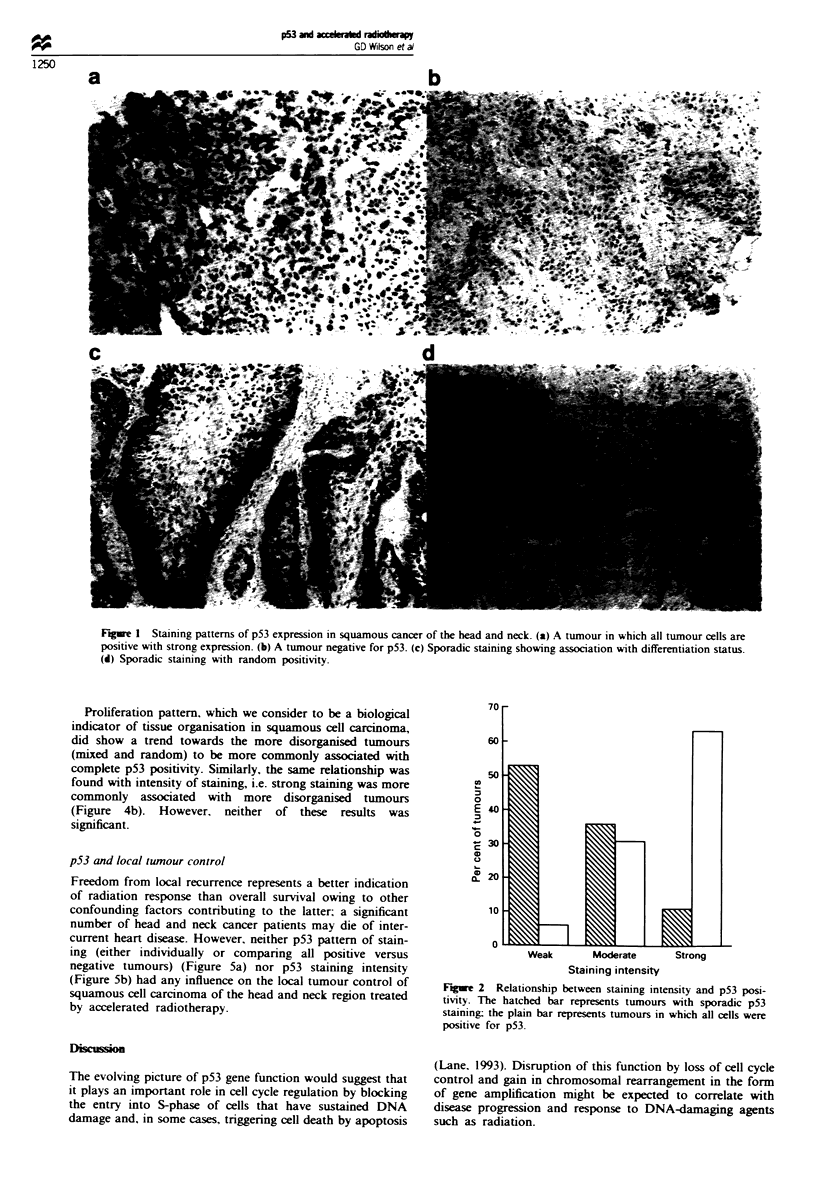
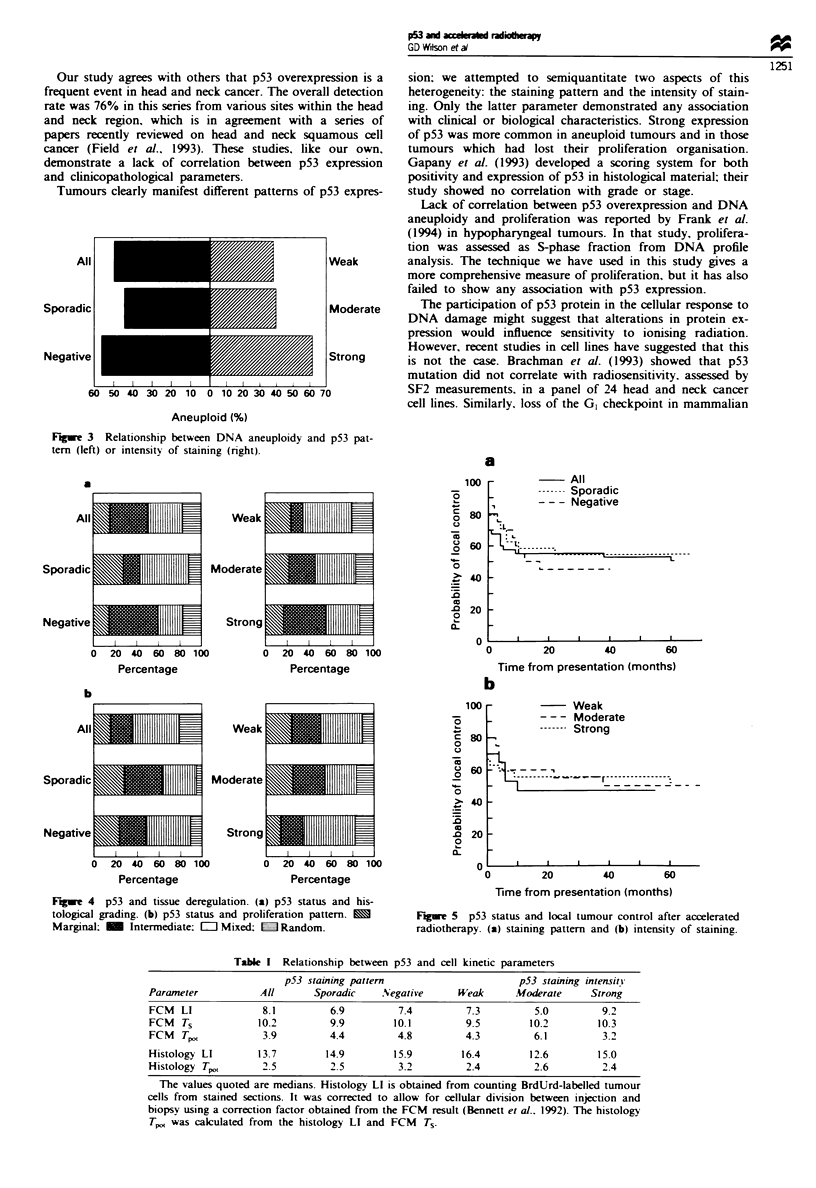
p53 status was investigated in 99 patients with squamous cell carcinoma of the head and neck region uniformly treated with accelerated radiotherapy and in whom tumour cell proliferation and DNA aneuploidy were assessed using bromodeoxyuridine (BrdUrd) incorporation and flow cytometry (FCM). Seventy-six percent of tumours were immunohistochemically positive for p53 protein, but heterogeneity was noticed both in the percentage of cells positive for p53 and in their level of expression. However, tumours which were either essentially all positive or all negative or showed sporadic positivity for p53 protein showed no differences in their level of aneuploidy, proliferation rate, tissue organisation or outcome with radiotherapy. There was a trend for those p53-positive tumours with the strongest expression to have more DNA aneuploidy and deregulation of proliferation organisation than weaker expressors; but there were no differences in proliferation rate or outcome of radiotherapy. These studies suggest that p53 protein stabilisation as assessed by immunohistochemistry does not have any major relationship with the biological characteristics and outcome of squamous cell cancer treated by accelerated radiotherapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. H., Wilson G. D., Dische S., Saunders M. I., Martindale C. A., Robinson B. M., O'Halloran A. E., Leslie M. D., Laing J. H. Tumour proliferation assessed by combined histological and flow cytometric analysis: implications for therapy in squamous cell carcinoma in the head and neck. Br J Cancer. 1992 Jun;65(6):870–878. doi: 10.1038/bjc.1992.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. O., Hakim J., Koch W., van der Riet P., Hruban R. H., Roa R. A., Correo R., Eby Y. J., Ruppert J. M., Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993 Oct 1;53(19):4477–4480. [PubMed] [Google Scholar]
- Brachman D. G., Beckett M., Graves D., Haraf D., Vokes E., Weichselbaum R. R. p53 mutation does not correlate with radiosensitivity in 24 head and neck cancer cell lines. Cancer Res. 1993 Aug 15;53(16):3667–3669. [PubMed] [Google Scholar]
- Cooper J. S., Pajak T. F., Rubin P., Tupchong L., Brady L. W., Leibel S. A., Laramore G. E., Marcial V. A., Davis L. W., Cox J. D. Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989 Sep;17(3):449–456. doi: 10.1016/0360-3016(89)90094-1. [DOI] [PubMed] [Google Scholar]
- Field J. K., Pavelic Z. P., Spandidos D. A., Stambrook P. J., Jones A. S., Gluckman J. L. The role of the p53 tumor suppressor gene in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1993 Oct;119(10):1118–1122. doi: 10.1001/archotol.1993.01880220064009. [DOI] [PubMed] [Google Scholar]
- Frank J. L., Bur M. E., Garb J. L., Kay S., Ware J. L., Sismanis A., Neifeld J. P. p53 tumor suppressor oncogene expression in squamous cell carcinoma of the hypopharynx. Cancer. 1994 Jan 1;73(1):181–186. doi: 10.1002/1097-0142(19940101)73:1<181::aid-cncr2820730131>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gapany M., Pavelic Z. P., Gapany S. R., Pavelic L., Li Y. G., Craven J. M., Jones H., Biddinger P., Stambrook P. J., Gluckman J. L. Relationship between immunohistochemically detectable p53 protein and prognostic factors in head and neck tumors. Cancer Detect Prev. 1993;17(3):379–386. [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P. Cancer. A death in the life of p53. Nature. 1993 Apr 29;362(6423):786–787. doi: 10.1038/362786a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. The regulation of p53 function: Steiner Award Lecture. Int J Cancer. 1994 Jun 1;57(5):623–627. doi: 10.1002/ijc.2910570502. [DOI] [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Maltzman W., Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984 Sep;4(9):1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees M., Homann N., Discher H., Andl T., Enders C., Herold-Mende C., Schuhmann A., Bosch F. X. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: a possible molecular basis for the development of multiple tumors. Cancer Res. 1993 Sep 15;53(18):4189–4196. [PubMed] [Google Scholar]
- SLAUGHTER D. P., SOUTHWICK H. W., SMEJKAL W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953 Sep;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Saunders M. I., Dische S., Grosch E. J., Fermont D. C., Ashford R. F., Maher E. J., Makepeace A. R. Experience with CHART. Int J Radiat Oncol Biol Phys. 1991 Aug;21(3):871–878. doi: 10.1016/0360-3016(91)90722-g. [DOI] [PubMed] [Google Scholar]
- Shin D. M., Kim J., Ro J. Y., Hittelman J., Roth J. A., Hong W. K., Hittelman W. N. Activation of p53 gene expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994 Jan 15;54(2):321–326. [PubMed] [Google Scholar]
- Slichenmyer W. J., Nelson W. G., Slebos R. J., Kastan M. B. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993 Sep 15;53(18):4164–4168. [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wilson G. D. Assessment of human tumour proliferation using bromodeoxyuridine--current status. Acta Oncol. 1991;30(8):903–910. doi: 10.3109/02841869109088242. [DOI] [PubMed] [Google Scholar]



