Abstract
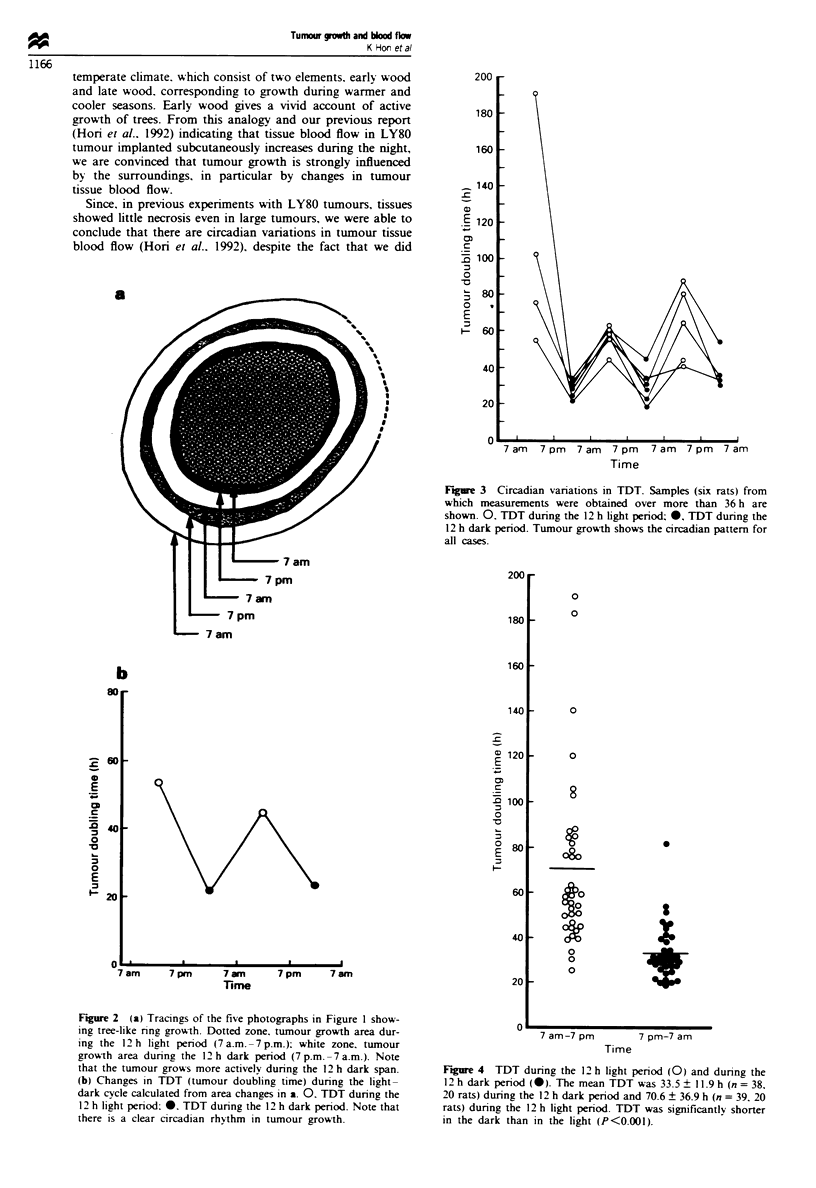
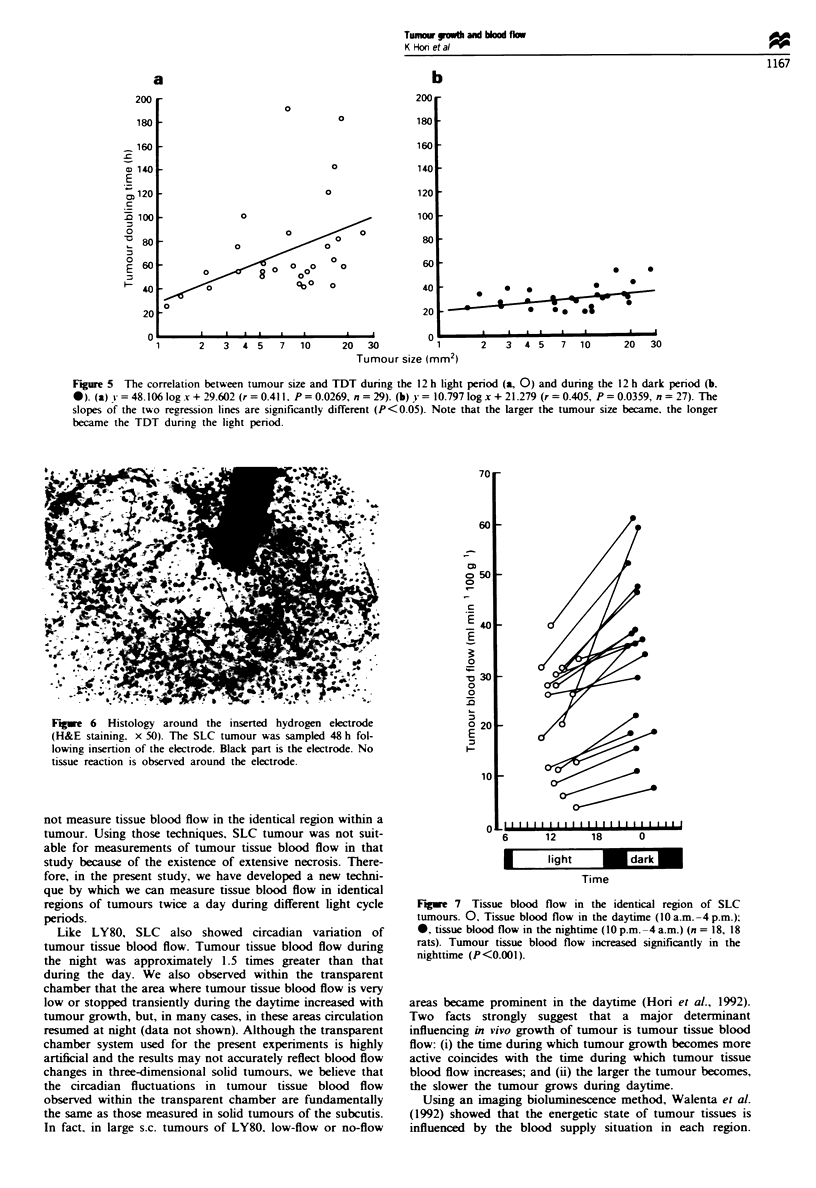
To determine whether tumour growth is influenced by circadian variations in tumour tissue blood flow, we measured changes in area doubling time of tumours (Sato lung carcinoma) within transparent chambers and changes in tissue blood flow of rat subcutaneous tumour during a light-dark cycle. Rats were subjected to an artificial light-dark cycle with light from 7 a.m. to 7 p.m. Tumour doubling times (TDTs) during the dark and the light spans were 33.5 +/- 11.9 h (n = 38, 20 rats) and 70.6 +/- 36.9 h (n = 39, 20 rats) respectively. The former was significantly shorter than the latter (P < 0.001). In addition, the larger the tumour became, the longer was the TDT during the light span (P < 0.05). Tumour tissue blood flow during the night (10 p.m.-4 a.m.) was approximately 1.5 times greater than that during the day (10 a.m.-4 p.m.). The time during which tumours actively grow and that during which tissue blood flow in tumours increases coincided. These results strongly suggest that tumour tissue blood flow is a determining influence on tumour proliferative activity and that tumour growth is influenced by circadian variations in tumour tissue blood flow.
Full text
PDF

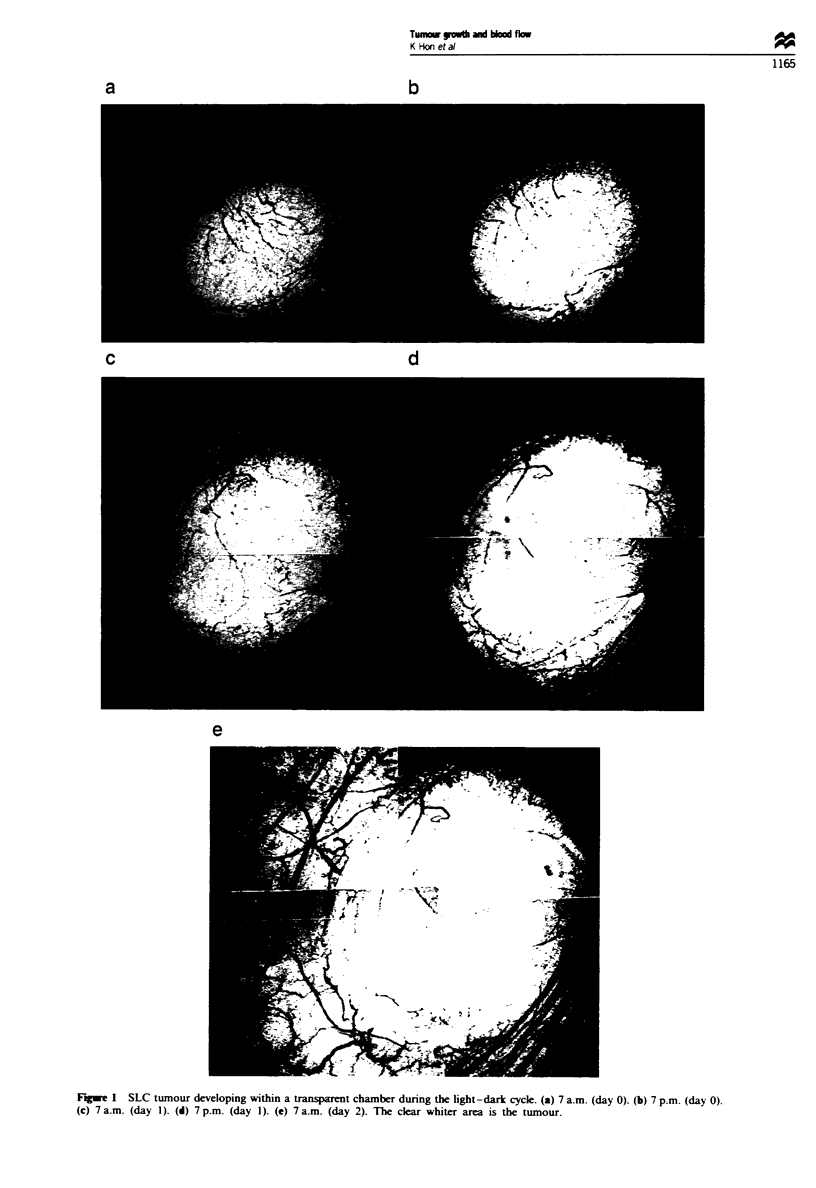

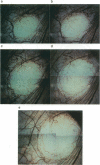
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUKLAND K., BOWER B. F., BERLINER R. W. MEASUREMENT OF LOCAL BLOOD FLOW WITH HYDROGEN GAS. Circ Res. 1964 Feb;14:164–187. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- Brammer I., Zywietz F., Jung H. Changes of histological and proliferative indices in the Walker carcinoma with tumour size and distance from blood vessel. Eur J Cancer. 1979 Nov;15(11):1329–1336. doi: 10.1016/0014-2964(79)90109-9. [DOI] [PubMed] [Google Scholar]
- Eddy H. A., Casarett G. W. Development of the vascular system in the hamster malignant neurilemmoma. Microvasc Res. 1973 Jul;6(1):63–82. doi: 10.1016/0026-2862(73)90007-1. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19(0):331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Gabbert H., Wagner R., Höhn P. The relation between tumor cell proliferation and vascularization in differentiated and undifferentiated colon carcinomas in the rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(1-2):119–131. doi: 10.1007/BF02890276. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972 Aug 1;136(2):261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Suzuki M., Tanda S., Saito S. In vivo analysis of tumor vascularization in the rat. Jpn J Cancer Res. 1990 Mar;81(3):279–288. doi: 10.1111/j.1349-7006.1990.tb02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Suzuki M., Tanda S., Saito S., Shinozaki M., Zhang Q. H. Circadian variation of tumor blood flow in rat subcutaneous tumors and its alteration by angiotensin II-induced hypertension. Cancer Res. 1992 Feb 15;52(4):912–916. [PubMed] [Google Scholar]
- Hori K., Suzuki M., Tanda S., Saito S., Shinozaki M., Zhang Q. H. Fluctuations in tumor blood flow under normotension and the effect of angiotensin II-induced hypertension. Jpn J Cancer Res. 1991 Nov;82(11):1309–1316. doi: 10.1111/j.1349-7006.1991.tb01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Zhang Q. H., Saito S., Tanda S., Li H. C., Suzuki M. Microvascular mechanisms of change in tumor blood flow due to angiotensin II, epinephrine, and methoxamine: a functional morphometric study. Cancer Res. 1993 Nov 15;53(22):5528–5534. [PubMed] [Google Scholar]
- Hrushesky W. J., Bjarnason G. A. Circadian cancer therapy. J Clin Oncol. 1993 Jul;11(7):1403–1417. doi: 10.1200/JCO.1993.11.7.1403. [DOI] [PubMed] [Google Scholar]
- Hrushesky W. J. Circadian timing of cancer chemotherapy. Science. 1985 Apr 5;228(4695):73–75. doi: 10.1126/science.3883493. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Schlenger K. H., Kloes M., Stohrer M., Vaupel P. Tumor blood flow: the principal modulator of oxidative and glycolytic metabolism, and of the metabolic micromilieu of human tumor xenografts in vivo. Int J Cancer. 1989 Aug 15;44(2):266–272. doi: 10.1002/ijc.2910440214. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R., Shymko R. M., Blumenfeld D., Braly P. S. Circadian gating of S phase in human ovarian cancer. Cancer Res. 1987 Dec 1;47(23):6267–6271. [PubMed] [Google Scholar]
- Porschen R., Classen S., Piontek M., Borchard F. Vascularization of carcinomas of the esophagus and its correlation with tumor proliferation. Cancer Res. 1994 Jan 15;54(2):587–591. [PubMed] [Google Scholar]
- Shimosato Y., Watanabe K. Enzymorphological observation on irradiated tumor, with a particular reference to acid hydrolase activity. I. Light microscopic study. Gan. 1967 Dec;58(6):541–550. [PubMed] [Google Scholar]
- Smaaland R., Lote K., Sothern R. B., Laerum O. D. DNA synthesis and ploidy in non-Hodgkin's lymphomas demonstrate intrapatient variation depending on circadian stage of cell sampling. Cancer Res. 1993 Jul 1;53(13):3129–3138. [PubMed] [Google Scholar]
- Suzuki M., Hori K., Saito S., Tanda S., Abe I., Sato H., Sato H. Functional characteristics of tumor vessels: selective increase in tumor blood flow. Sci Rep Res Inst Tohoku Univ Med. 1989 Dec;36(1-4):37–45. [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Fortmeyer H. P., Runkel S., Kallinowski F. Blood flow, oxygen consumption, and tissue oxygenation of human breast cancer xenografts in nude rats. Cancer Res. 1987 Jul 1;47(13):3496–3503. [PubMed] [Google Scholar]
- Walenta S., Dellian M., Goetz A. E., Kuhnle G. E., Mueller-Klieser W. Pixel-to-pixel correlation between images of absolute ATP concentrations and blood flow in tumours. Br J Cancer. 1992 Dec;66(6):1099–1102. doi: 10.1038/bjc.1992.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin T., Soussi B., Idström J. P., Lindnér P., Edström S., Lydén E., Gustavsson B., Hafström L., Lundholm K. Energetics of nutrition and polyamine-related tumor growth alterations in experimental cancer. Br J Cancer. 1993 Oct;68(4):662–667. doi: 10.1038/bjc.1993.405. [DOI] [PMC free article] [PubMed] [Google Scholar]