Abstract
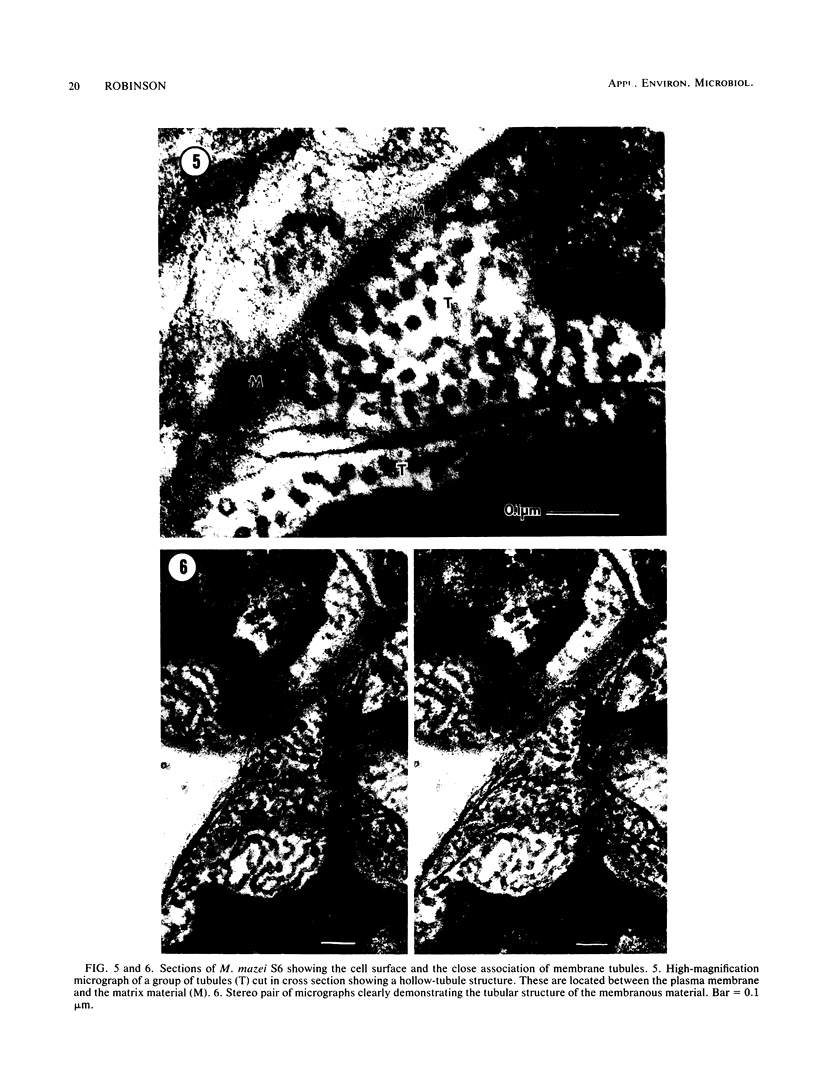
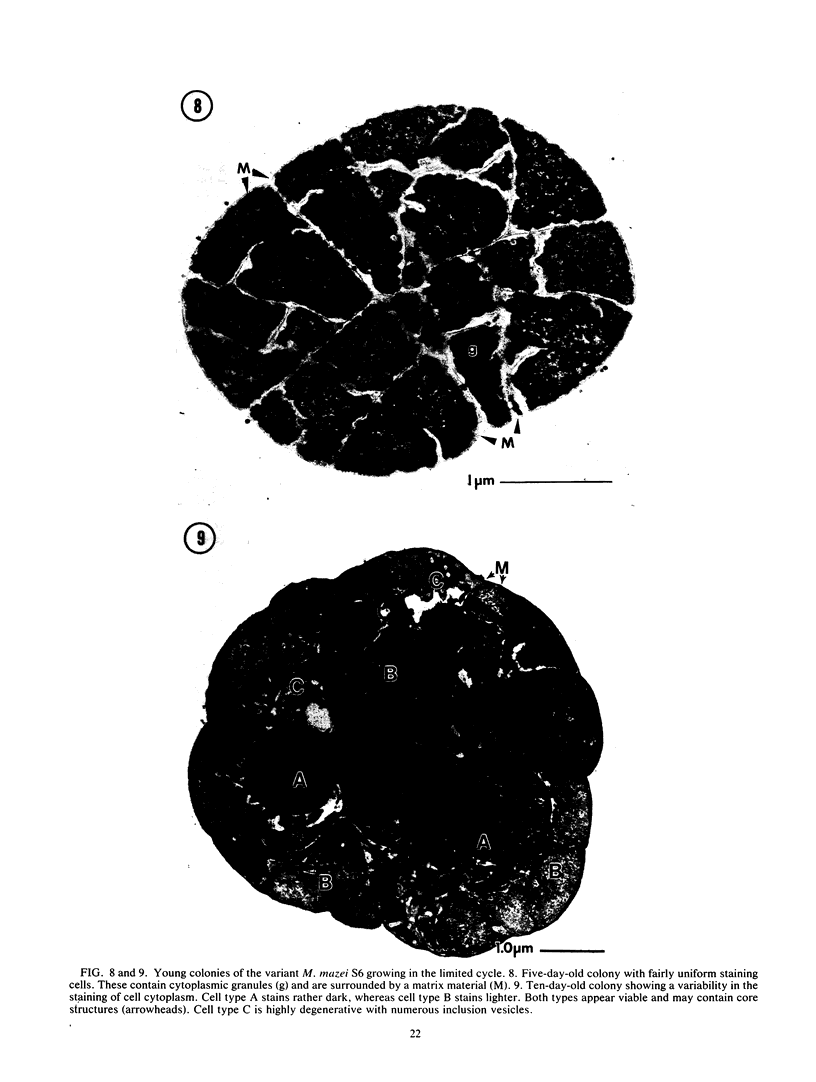
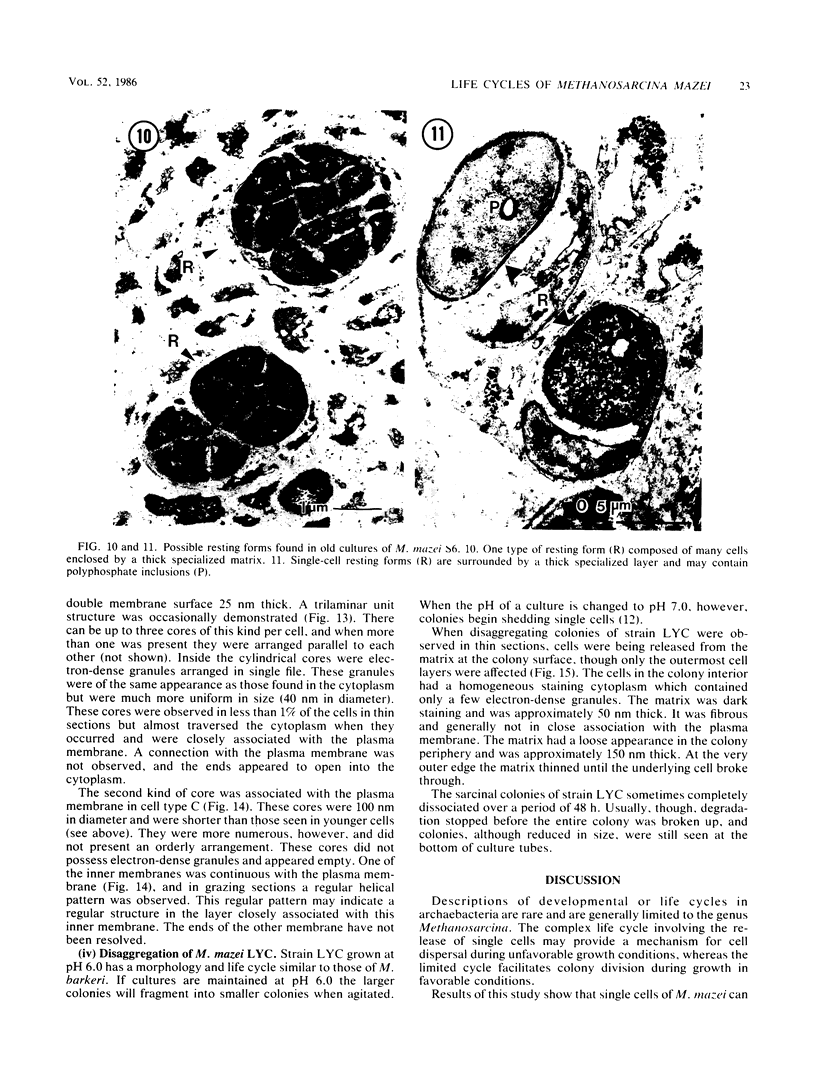
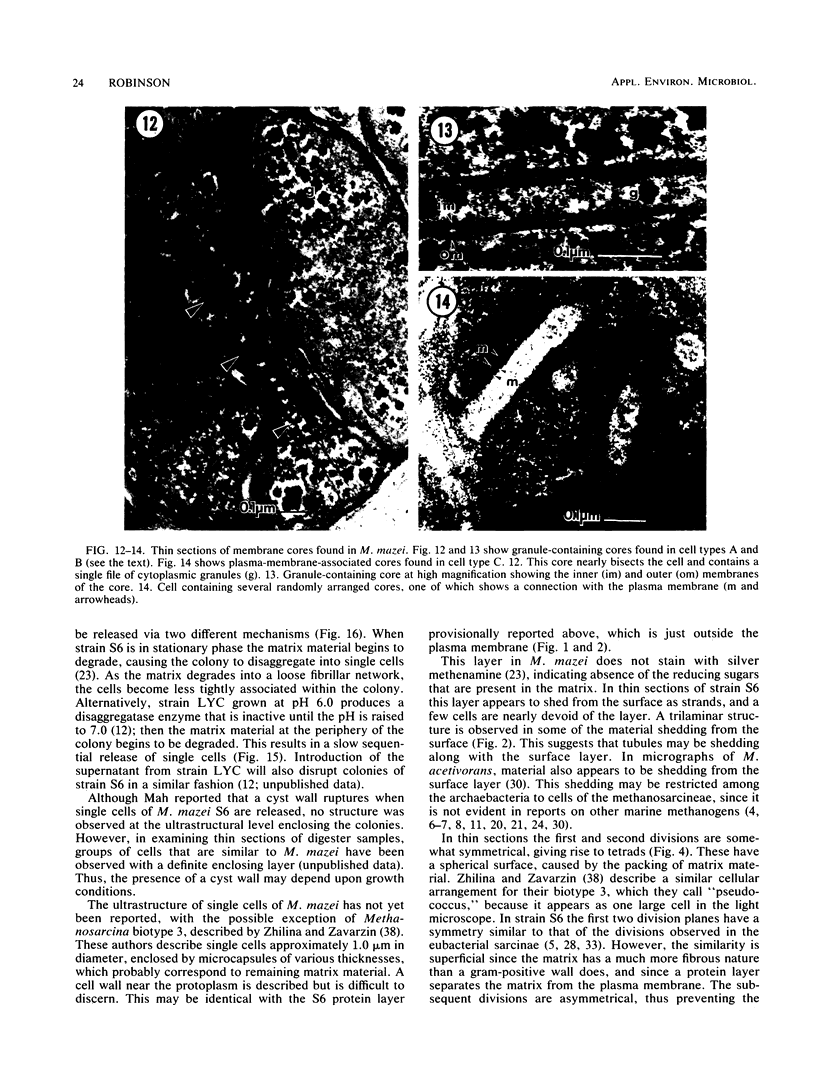
Methanosarcina mazei S6 and LYC were used to study the structure and differentiation of the aggregating methanogens. Cultures harvested under various conditions are described at the ultrastructural level. Cells of strain S6 are enclosed by a layer 12 nm thick in contact with the plasma membrane. In sarcinal colonies, cells are held in close association by a fibrous matrix up to 60 nm thick. Colony maturation was examined in strain S6 over a period of 1 year. Changes occurred in the shape and staining of individual cells. Also, various inclusion bodies were observed that either persist throughout colony maturation or are only found at certain growth stages. Two types of cores that are composed of double membranes in M. mazei S6 are described. One has a 90-nm diameter and contains electron-dense granules similar to those found in the cytoplasm. The other core type does not contain granules, is more numerous, and is found in older cultures. Two life cycles are described for M. mazei based on electron microscope examinations. A complex life cycle involving the release of single cells is described with two variations for strains S6 and LYC. When released cells of strain S6 are placed in fresh medium they can repeat the cycle. In addition, a limited cycle is described for both strains of M. mazei. This limited cycle contains the only sarcinal morphotypes observed in M. barkeri. When M. mazei S6 remains in the limited cycle and does not disaggregate in stationary phase, several types of possible resting forms are found.
Full text
PDF

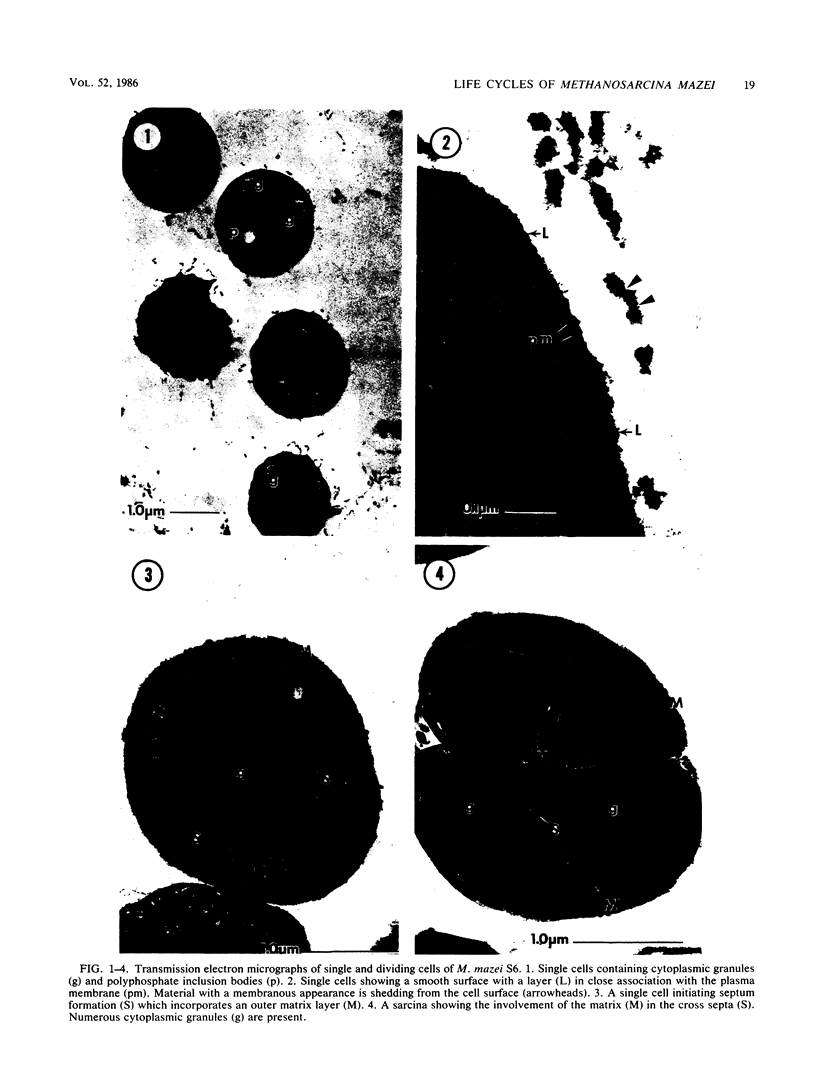



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. The structure and development of mesosomes studied in Bacillus licheniformis strain 6346. J Ultrastruct Res. 1972 Jan;38(1):113–133. doi: 10.1016/s0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., Bleiweis A. S. Ultrastructural, physiological, and cytochemical characterization of cores in group D streptococci. J Bacteriol. 1977 Jan;129(1):445–456. doi: 10.1128/jb.129.1.445-456.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. J., Mah R. A. Isolation and characterization of an h(2)-oxidizing thermophilic methanogen. Appl Environ Microbiol. 1983 Jan;45(1):265–274. doi: 10.1128/aem.45.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Canale-Parola E. Fine structure of Sarcina maxima and Sarcina ventriculi. J Bacteriol. 1967 Jan;93(1):399–410. doi: 10.1128/jb.93.1.399-410.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Boone D. R., Sleat R., Mah R. A. Methanosarcina mazei LYC, a New Methanogenic Isolate Which Produces a Disaggregating Enzyme. Appl Environ Microbiol. 1985 Mar;49(3):608–613. doi: 10.1128/aem.49.3.608-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Meister N., Moor H. Freezing in a propane jet and its application in freeze-fracturing. Mikroskopie. 1980 Sep;36(5-6):129–140. [PubMed] [Google Scholar]
- Ollivier B., Lombardo A., Garcia J. L. Isolation and characterization of a new thermophilic Methanosarcina strain (strain MP). Ann Microbiol (Paris) 1984 Sep-Oct;135B(2):187–198. doi: 10.1016/s0769-2609(84)80026-5. [DOI] [PubMed] [Google Scholar]
- Rivard C. J., Henson J. M., Thomas M. V., Smith P. H. Isolation and Characterization of Methanomicrobium paynteri sp. nov., a Mesophilic Methanogen Isolated from Marine Sediments. Appl Environ Microbiol. 1983 Aug;46(2):484–490. doi: 10.1128/aem.46.2.484-490.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W., Akin D. E., Nordstedt R. A., Thomas M. V., Aldrich H. C. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl Environ Microbiol. 1984 Jul;48(1):127–136. doi: 10.1128/aem.48.1.127-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W., Aldrich H. C., Hurst S. F., Bleiweis A. S. Role of the Cell Surface of Methanosarcina mazei in Cell Aggregation. Appl Environ Microbiol. 1985 Feb;49(2):321–327. doi: 10.1128/aem.49.2.321-327.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Sowers K. R., Baron S. F., Ferry J. G. Methanosarcina acetivorans sp. nov., an Acetotrophic Methane-Producing Bacterium Isolated from Marine Sediments. Appl Environ Microbiol. 1984 May;47(5):971–978. doi: 10.1128/aem.47.5.971-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Ferry J. G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol. 1983 Feb;45(2):684–690. doi: 10.1128/aem.45.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Horne R. W., Glauert A. M. The fine structure of Micrococcus radiodurans. Arch Mikrobiol. 1965 Jul 20;51(3):267–289. doi: 10.1007/BF00408143. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]