Abstract
The cellular response, in terms of cell cycle arrest(s) and apoptosis, to radiation-induced DNA damage was studied. Experiments were performed on both mitogen-stimulated and resting peripheral blood lymphocytes (PBLs) from normal and cancer-prone (C-P) individuals. The C-P individuals comprised three patients carrying germline p53 mutations and three members of two families apparently without such mutations, but with an inherited defect which results in p53 deregulation as shown by high levels of stabilised p53 protein in normal tissues. Interestingly, mitogen-stimulated PBL, from both normal and C-P individuals failed to demonstrate a G1 arrest after gamma radiation. However, a clear difference was seen in the apoptotic response to DNA damage, of PBL from normal and C-P individuals; PBLs from C-P individuals with inherited p53-related defects had a reduced apoptotic response (P = 0.0003). There was a wide margin of separation, with no overlap between the two groups, supporting the possibility of using this altered apoptotic response as a screening test. This simple and rapid procedure could be used to identify those individuals in a C-P family who carry germline p53-related defects. The method appears to detect both individuals with p53 mutations and those apparently without mutations but with other p53-related defects.
Full text
PDF

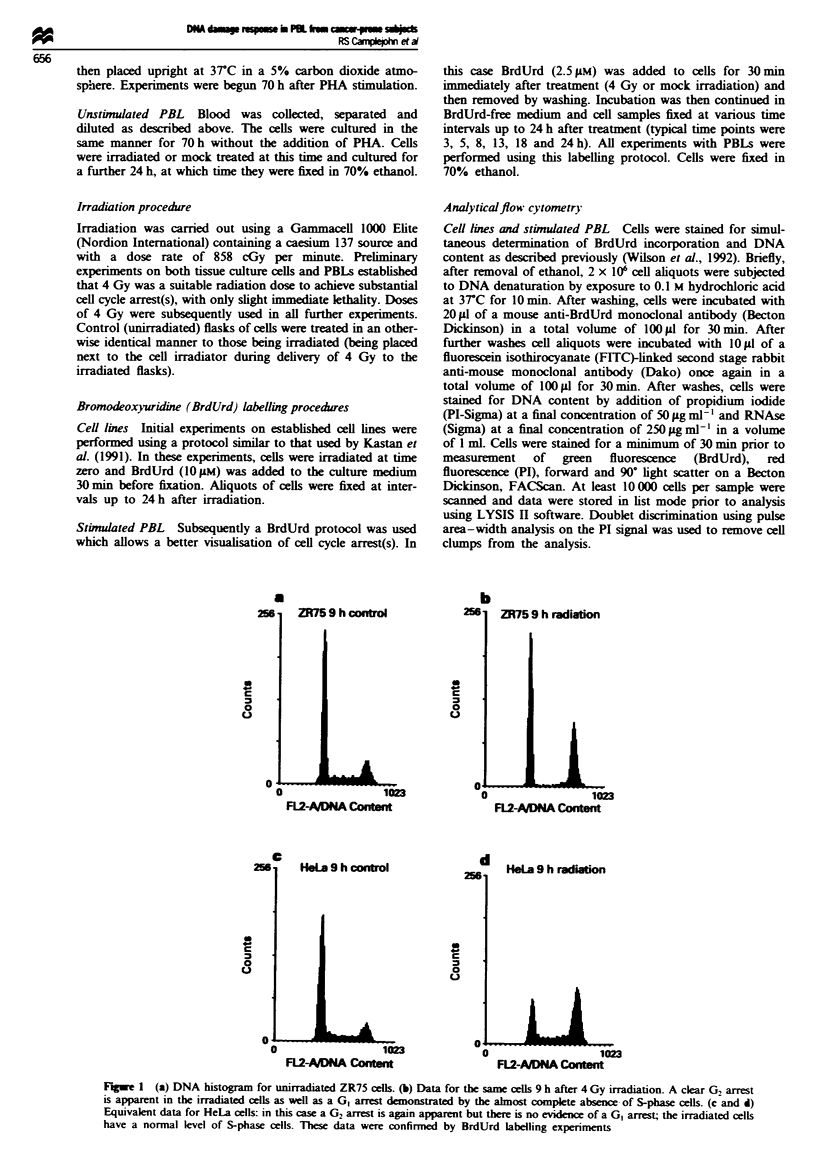


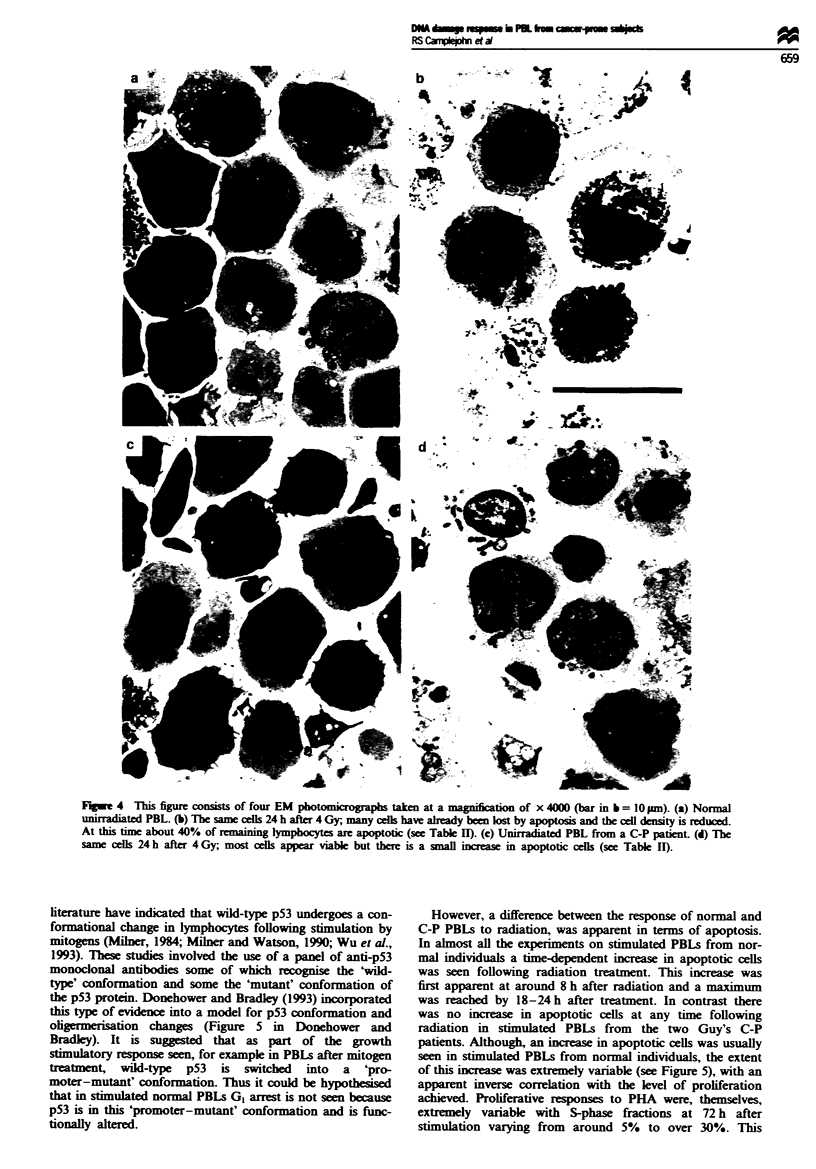
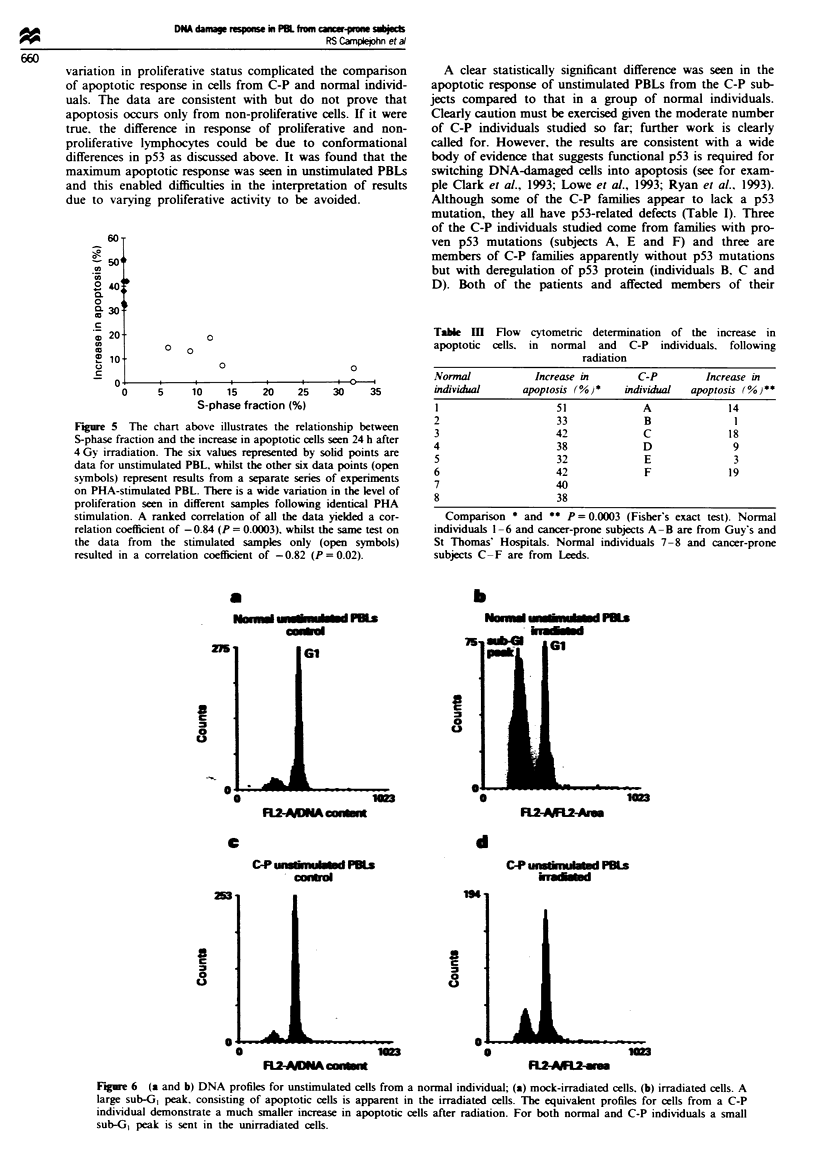


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Barton C. M., Staddon S. L., Hughes C. M., Hall P. A., O'Sullivan C., Klöppel G., Theis B., Russell R. C., Neoptolemos J., Williamson R. C. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991 Dec;64(6):1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J. M., Hartley A. L., Tricker K. J., Prosser J., Condie A., Kelsey A. M., Harris M., Jones P. H., Binchy A., Crowther D. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res. 1994 Mar 1;54(5):1298–1304. [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Crook T., Wrede D., Vousden K. H. p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene. 1991 May;6(5):873–875. [PubMed] [Google Scholar]
- Donehower L. A., Bradley A. The tumor suppressor p53. Biochim Biophys Acta. 1993 Aug 23;1155(2):181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- Eeles R. A. Predictive testing for germline mutations in the p53 gene: are all the questions answered? Eur J Cancer. 1993;29A(10):1361–1365. doi: 10.1016/0959-8049(93)90001-v. [DOI] [PubMed] [Google Scholar]
- Fritsche M., Haessler C., Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993 Feb;8(2):307–318. [PubMed] [Google Scholar]
- Garber J. E., Goldstein A. M., Kantor A. F., Dreyfus M. G., Fraumeni J. F., Jr, Li F. P. Follow-up study of twenty-four families with Li-Fraumeni syndrome. Cancer Res. 1991 Nov 15;51(22):6094–6097. [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992 Nov 13;71(4):543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lee J. M., Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr, Mulvihill J. J., Blattner W. A., Dreyfus M. G., Tucker M. A., Miller R. W. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988 Sep 15;48(18):5358–5362. [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- MacGeoch C., Turner G., Bobrow L. G., Barnes D. M., Bishop D. T., Spurr N. K. Heterogeneity in Li-Fraumeni families: p53 mutation analysis and immunohistochemical staining. J Med Genet. 1995 Mar;32(3):186–190. doi: 10.1136/jmg.32.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Milner J. Different forms of p53 detected by monoclonal antibodies in non-dividing and dividing lymphocytes. Nature. 1984 Jul 12;310(5973):143–145. doi: 10.1038/310143a0. [DOI] [PubMed] [Google Scholar]
- Milner J., Watson J. V. Addition of fresh medium induces cell cycle and conformation changes in p53, a tumour suppressor protein. Oncogene. 1990 Nov;5(11):1683–1690. [PubMed] [Google Scholar]
- Ormerod M. G., Collins M. K., Rodriguez-Tarduchy G., Robertson D. Apoptosis in interleukin-3-dependent haemopoietic cells. Quantification by two flow cytometric methods. J Immunol Methods. 1992 Aug 30;153(1-2):57–65. doi: 10.1016/0022-1759(92)90305-d. [DOI] [PubMed] [Google Scholar]
- Ryan J. J., Danish R., Gottlieb C. A., Clarke M. F. Cell cycle analysis of p53-induced cell death in murine erythroleukemia cells. Mol Cell Biol. 1993 Jan;13(1):711–719. doi: 10.1128/mcb.13.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., Camplejohn R. S., Martindale C. A., Brock A., Lane D. P., Barnes D. M. Flow cytometric characterisation of proliferating cell nuclear antigen using the monoclonal antibody PC10. Eur J Cancer. 1992;28A(12):2010–2017. doi: 10.1016/0959-8049(92)90250-6. [DOI] [PubMed] [Google Scholar]
- Wu J., Wang M., Li X., Sheng Y. Conformation changes of p53 proteins in regulation of murine T lymphocyte proliferation. Cell Mol Biol Res. 1993;39(1):27–31. [PubMed] [Google Scholar]
- Wu X., Levine A. J. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Grunwald D., Wilder S., Kimchi A., May E., Lawrence J. J., May P., Oren M. p53-mediated cell death: relationship to cell cycle control. Mol Cell Biol. 1993 Mar;13(3):1415–1423. doi: 10.1128/mcb.13.3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]