Abstract
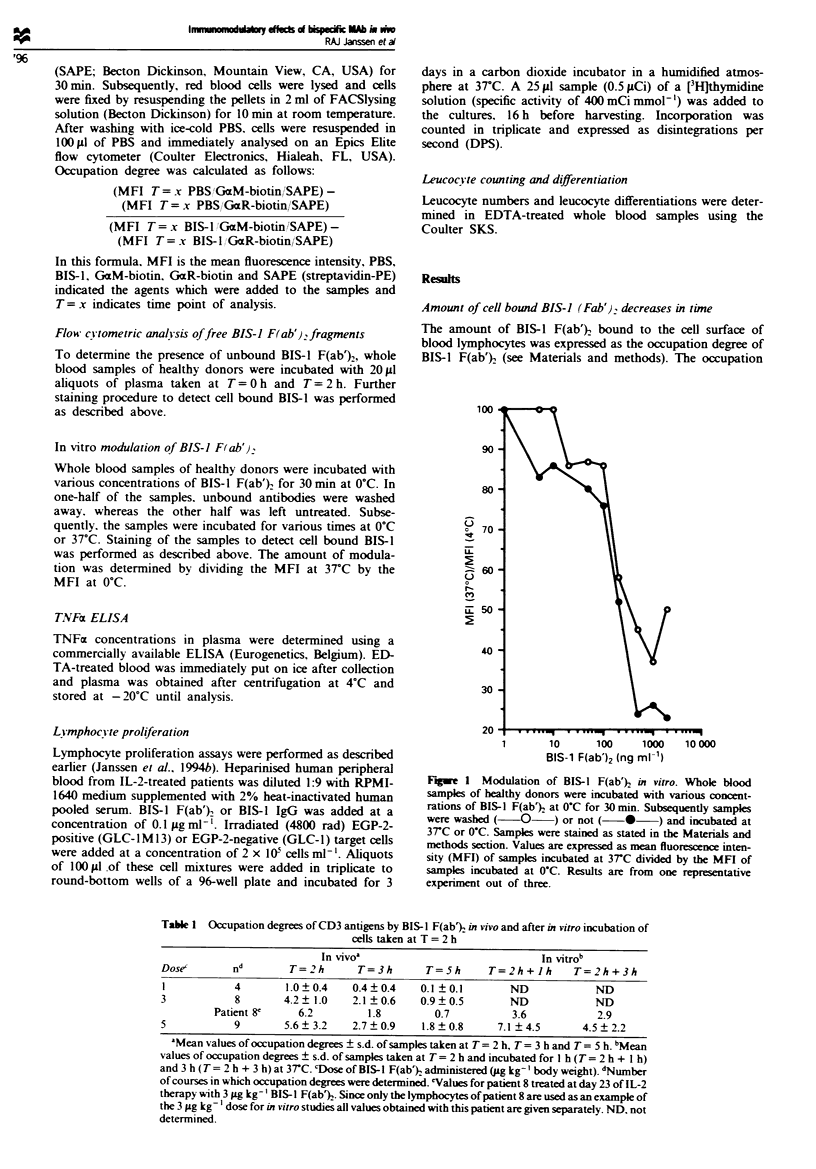
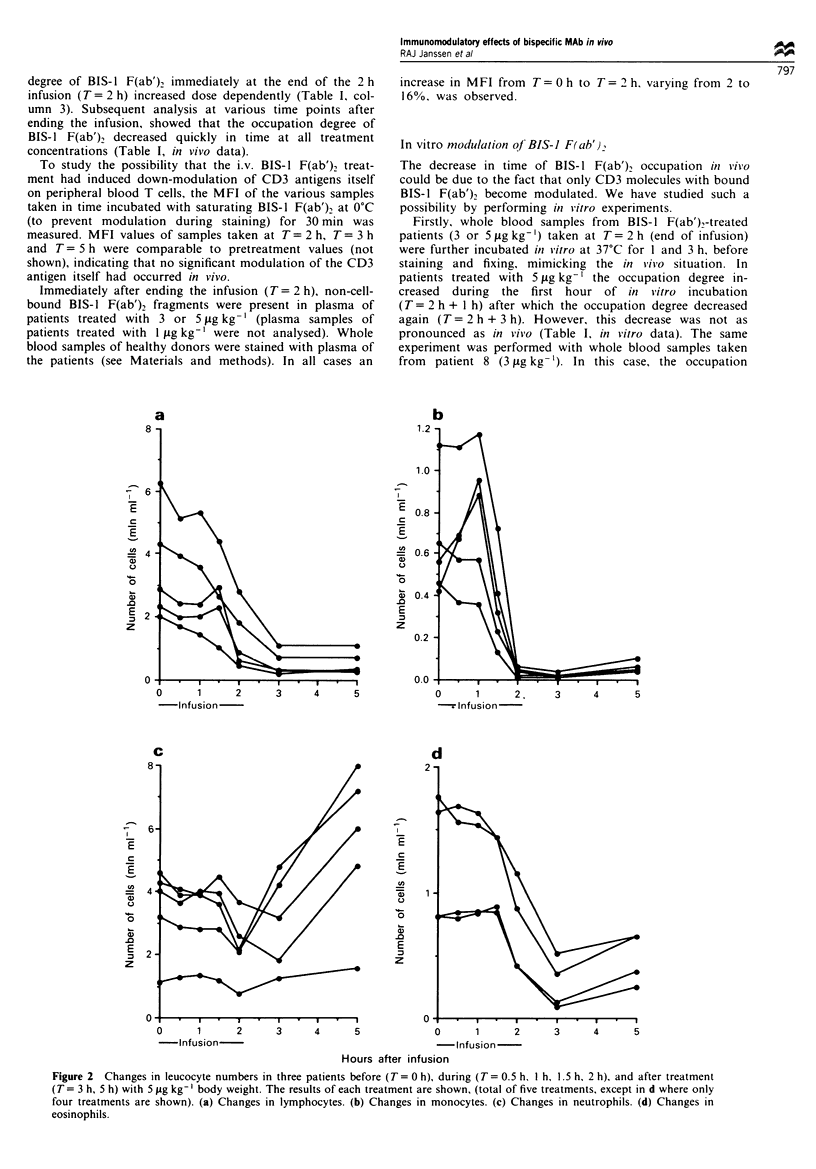
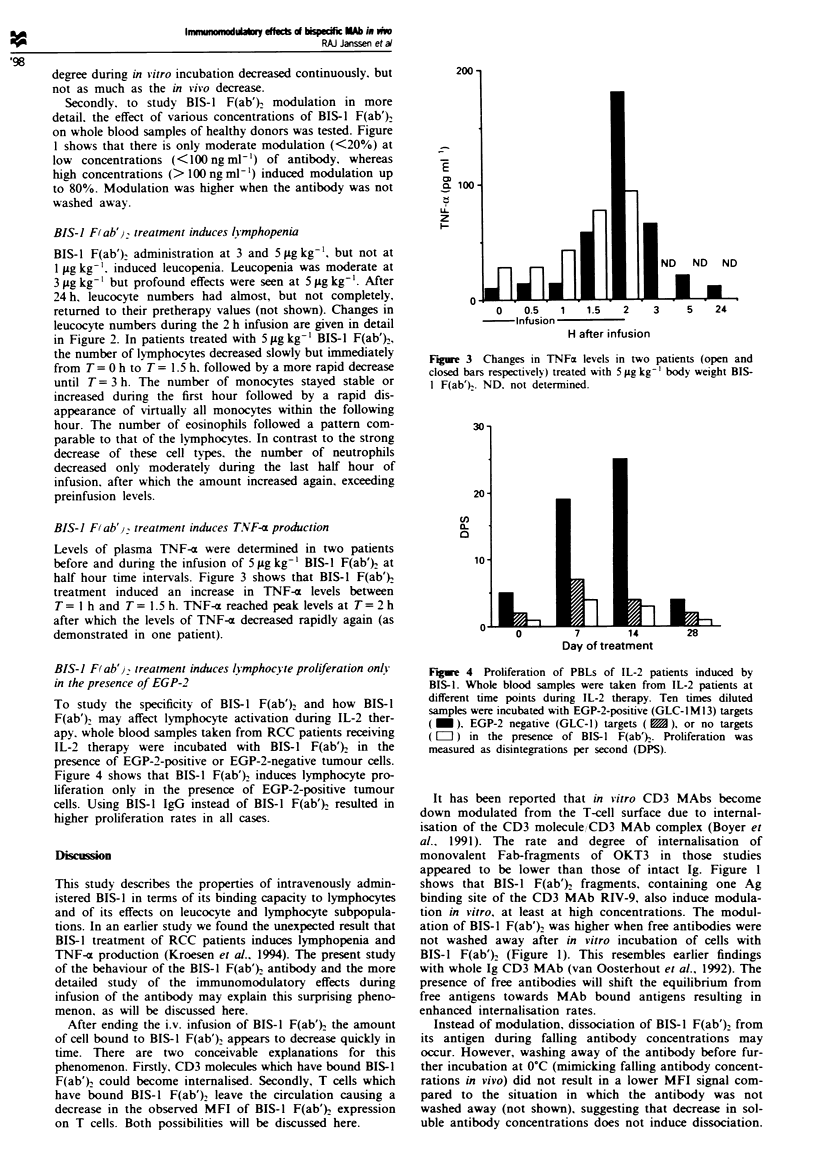
We report the immunomodulatory effects of an intravenous treatment with F(ab')2 fragments of the bispecific monoclonal antibody BIS-1 during subcutaneous recombinant interleukin 2 (rIL-2) therapy of renal cell cancer (RCC) patients. BIS-1 is directed against both the CD3 antigen on T cells and the EGP-2 molecule on carcinoma cells and some normal epithelia. The amount of BIS-1 F(ab')2 bound to peripheral blood lymphocytes (PBLs) increased dose-dependently. This occupation degree was highest at the end of the 2 h infusion and rapidly decreased subsequently. During the first hour of BIS-1 F(ab')2 infusion the number of PBLs decreased slowly. This was followed by an increase in serum tumour necrosis factor alpha (TNF-alpha) concentrations and a rapid decrease in the numbers of peripheral blood lymphocytes, monocytes and eosinophils. In our view, the most likely explanation for the observed decrease in occupation degree of BIS-1 F(ab')2 and the rise in TNF-alpha levels is based on the assumption that BIS-1-carrying T cells leave the circulation. The CD3 antigens on these extravasated T cells become cross-linked by EGP-2 antigens, inducing TNF-alpha secretion. This results in an enhanced decrease in the numbers of PBLs, monocytes and eosinophils. These preliminary results suggest that BIS-1 F(ab')2 treatment during IL-2 therapy may induce local T-cell activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer C., Auphan N., Luton F., Malburet J. M., Barad M., Bizozzero J. P., Reggio H., Schmitt-Verhulst A. M. T cell receptor/CD3 complex internalization following activation of a cytolytic T cell clone: evidence for a protein kinase C-independent staurosporine-sensitive step. Eur J Immunol. 1991 Jul;21(7):1623–1634. doi: 10.1002/eji.1830210707. [DOI] [PubMed] [Google Scholar]
- De Leij L., Helrich W., Stein R., Mattes M. J. SCLC-cluster-2 antibodies detect the pancarcinoma/epithelial glycoprotein EGP-2. Int J Cancer Suppl. 1994;8:60–63. doi: 10.1002/ijc.2910570713. [DOI] [PubMed] [Google Scholar]
- Janssen R. A., Buter J., Straatsma E., Heijn A. A., Sleijfer D. T., de Vries E. G., Mulder N. H., The T. H., de Leij L. HLA-Dr-expressing CD8bright cells are only temporarily present in the circulation during subcutaneous recombinant interleukin-2 therapy in renal cell carcinoma patients. Cancer Immunol Immunother. 1993;36(3):198–204. doi: 10.1007/BF01741092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R. A., Heijn A. A., The T. H., de Leij L. Poor induction of interleukin-2 receptor expression on CD8bright+ cells in whole blood cell cultures with CD3 mAb. Implications for immunotherapy with CD3 mAb. Cancer Immunol Immunother. 1994 Jan;38(1):53–60. doi: 10.1007/BF01517170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R. A., Mulder N. H., The T. H., de Leij L. The immunobiological effects of interleukin-2 in vivo. Cancer Immunol Immunother. 1994 Oct;39(4):207–216. doi: 10.1007/BF01525983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroesen B. J., Buter J., Sleijfer D. T., Janssen R. A., van der Graaf W. T., The T. H., de Leij L., Mulder N. H. Phase I study of intravenously applied bispecific antibody in renal cell cancer patients receiving subcutaneous interleukin 2. Br J Cancer. 1994 Oct;70(4):652–661. doi: 10.1038/bjc.1994.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleijfer D. T., Janssen R. A., Buter J., de Vries E. G., Willemse P. H., Mulder N. H. Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal cell cancer on an outpatient basis. J Clin Oncol. 1992 Jul;10(7):1119–1123. doi: 10.1200/JCO.1992.10.7.1119. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Lee D. J., Lindgren C. G., Benz L. A., Collins C., Shuman W. P., Levitt D., Fefer A. Influence of schedule of interleukin 2 administration on therapy with interleukin 2 and lymphokine activated killer cells. Cancer Res. 1989 Jan 1;49(1):235–240. [PubMed] [Google Scholar]
- Tibben J. G., Boerman O. C., Claessens R. A., Corstens F. H., van Deuren M., de Mulder P. H., van der Meer J. W., Keijser K. G., Massuger L. F. Cytokine release in an ovarian carcinoma patient following intravenous administration of bispecific antibody OC/TR F(ab')2. J Natl Cancer Inst. 1993 Jun 16;85(12):1003–1004. doi: 10.1093/jnci/85.12.1003. [DOI] [PubMed] [Google Scholar]
- Woodle E. S., Thistlethwaite J. R., Ghobrial I. A., Jolliffe L. K., Stuart F. P., Bluestone J. A. OKT3 F(ab')2 fragments--retention of the immunosuppressive properties of whole antibody with marked reduction in T cell activation and lymphokine release. Transplantation. 1991 Aug;52(2):354–360. doi: 10.1097/00007890-199108000-00033. [DOI] [PubMed] [Google Scholar]
- Yoshino I., Yano T., Murata M., Ishida T., Sugimachi K., Kimura G., Nomoto K. Cytolytic potential of peripheral blood T-lymphocytes following adoptive immunotherapy with lymphokine-activated killer cells and low-dose interleukin 2. Cancer Res. 1991 Mar 1;51(5):1494–1498. [PubMed] [Google Scholar]


