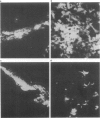
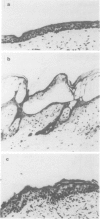
Abstract
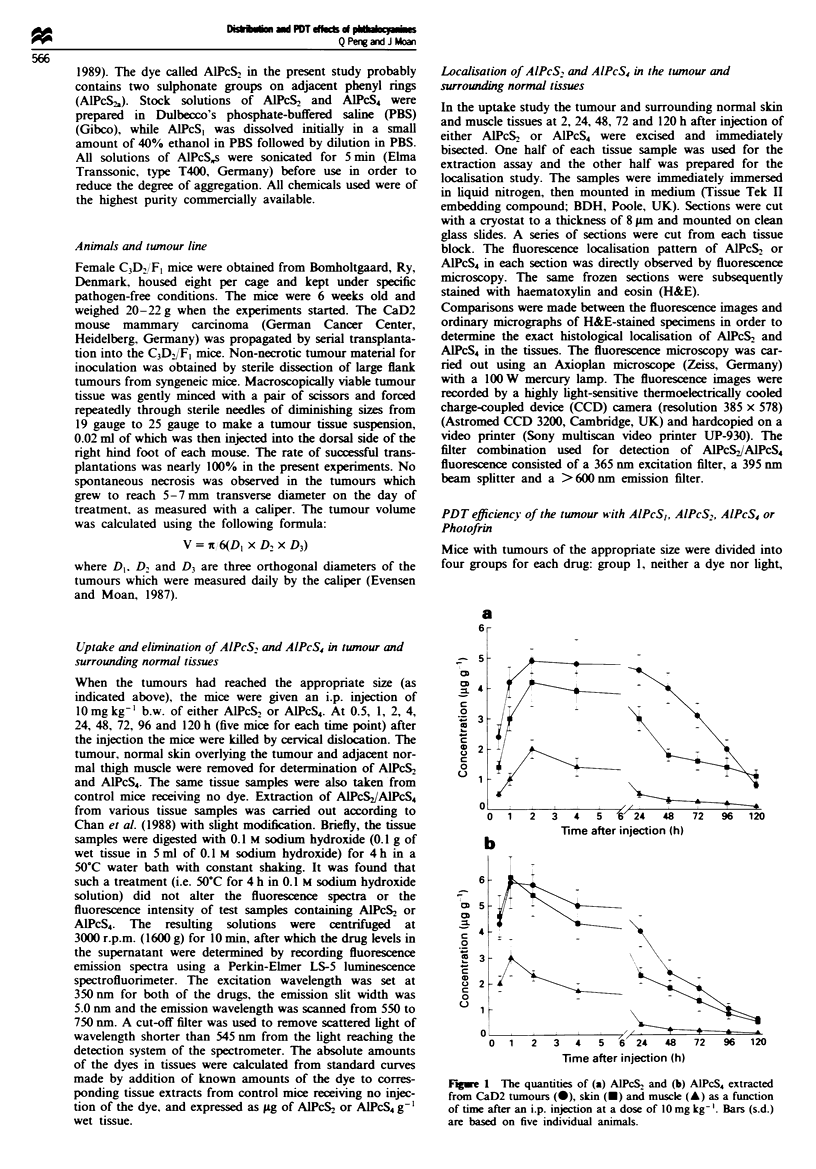
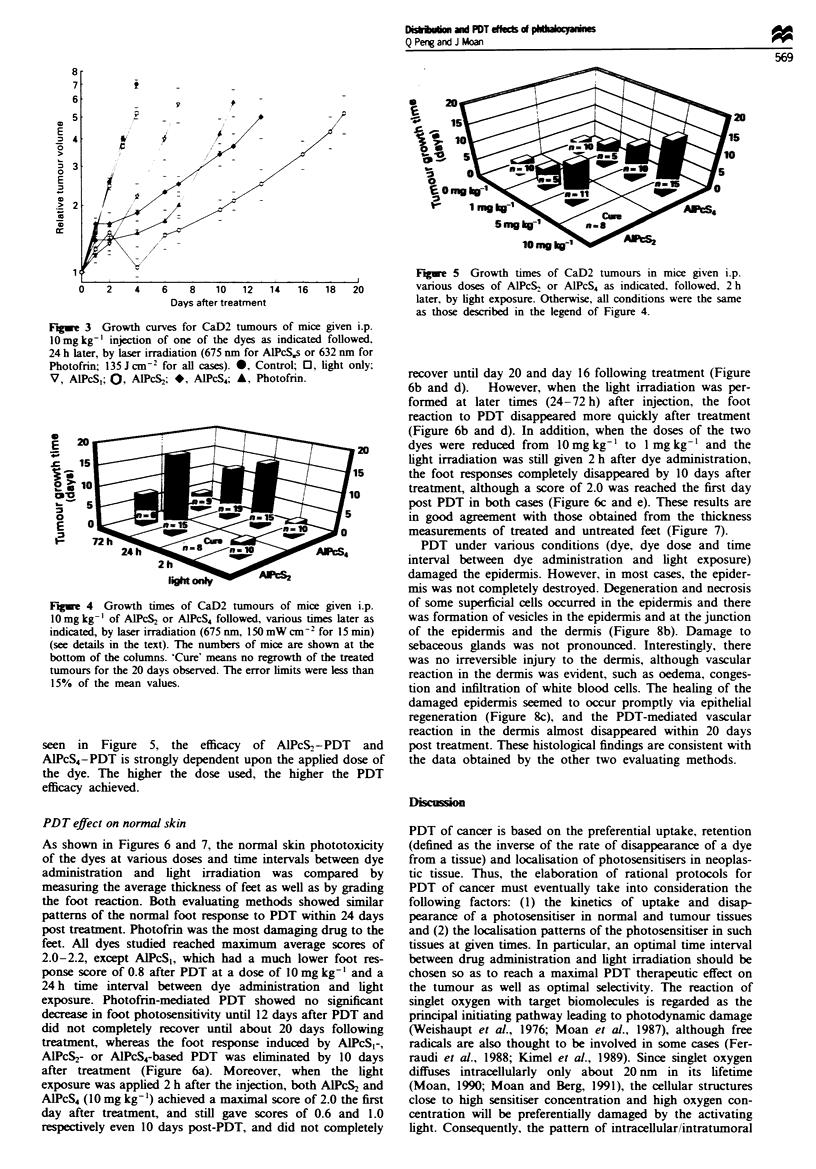
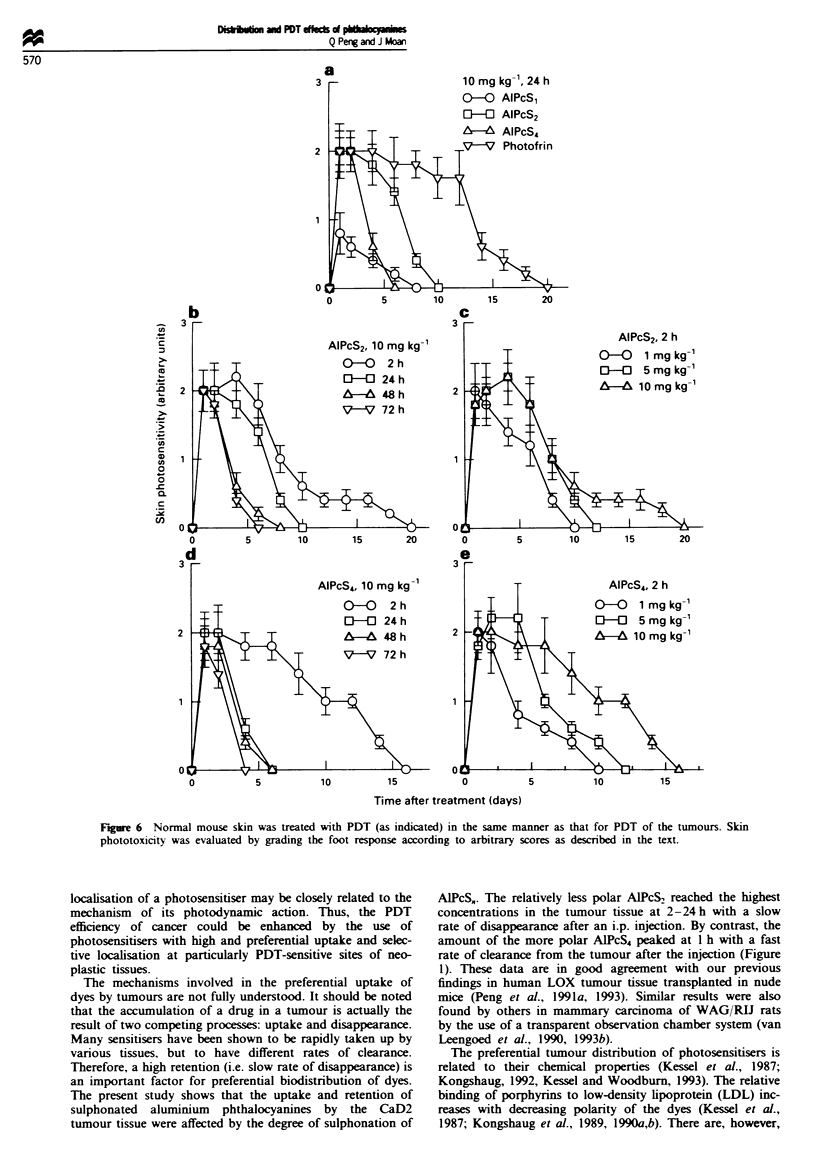
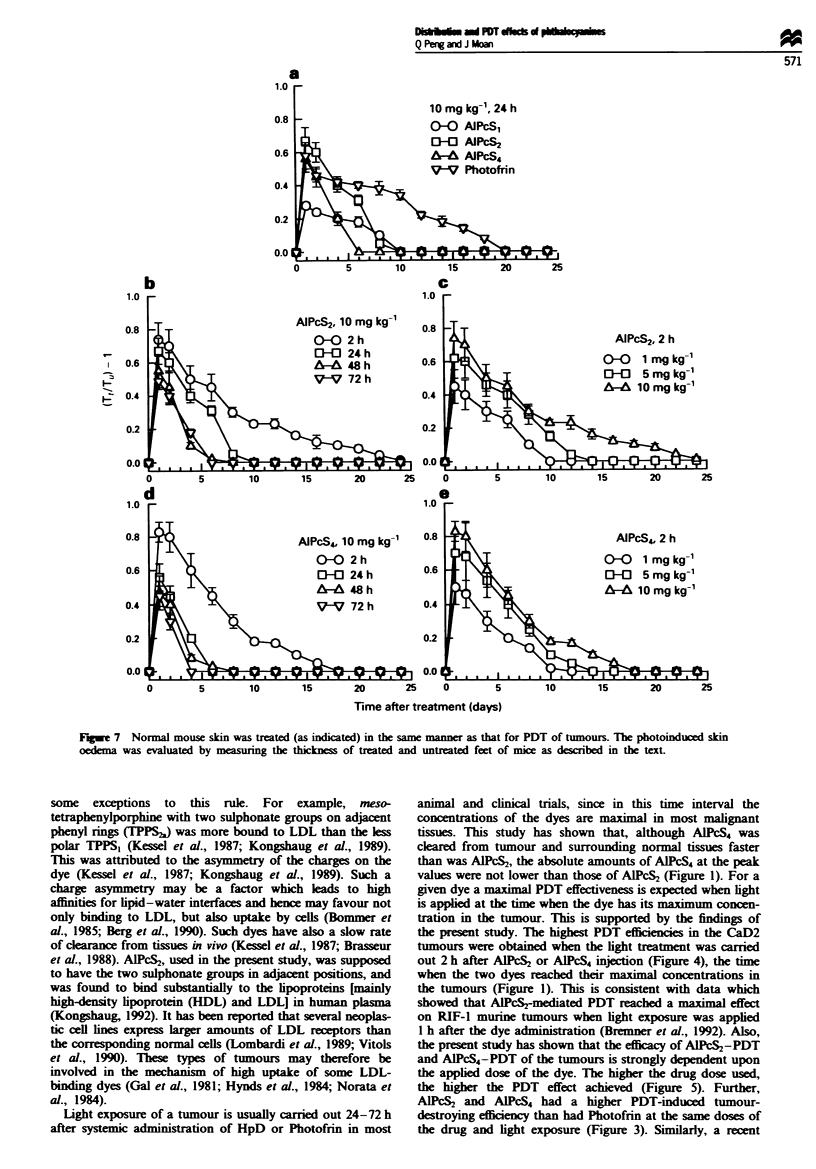
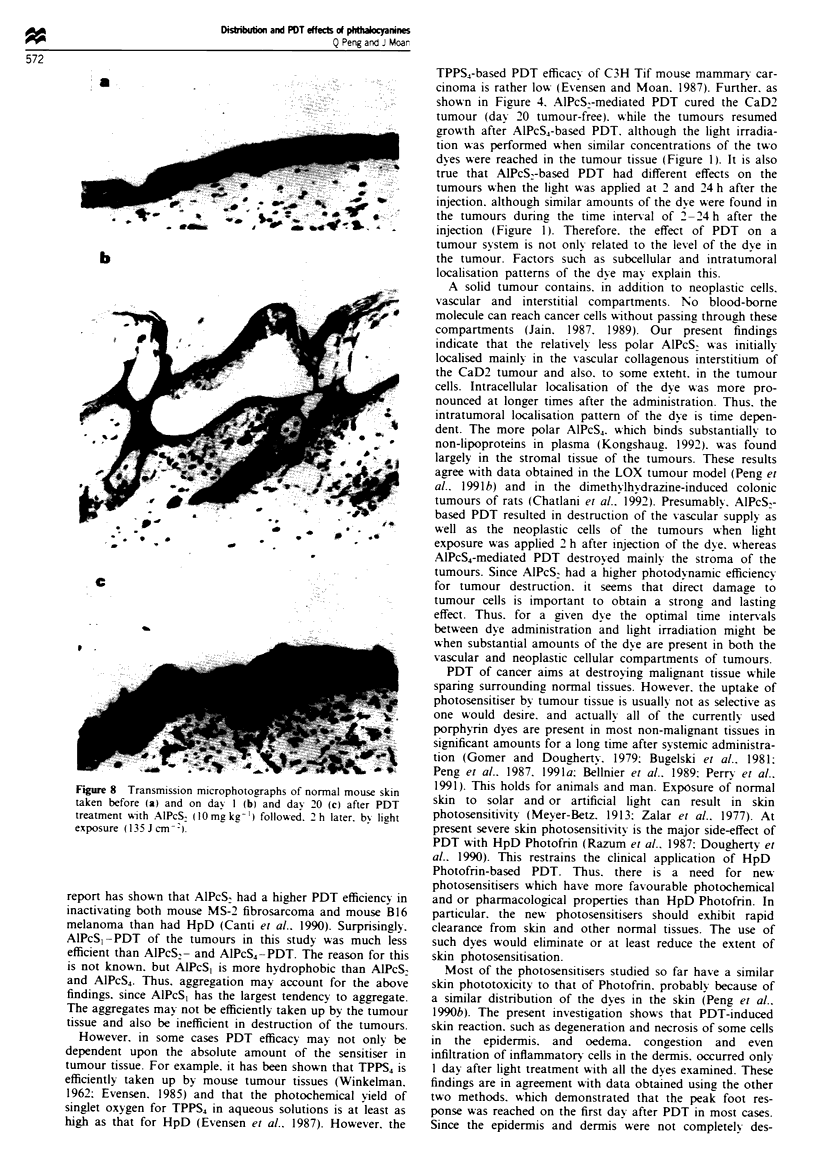
A chemical extraction assay and fluorescence microscopy incorporating a light-sensitive thermoelectrically cooled charge-coupled device (CCD) camera was used to study the kinetics of uptake, retention and localisation of disulphonated aluminium phthalocyanine (A1PcS2) and tetrasulphonated aluminium phthalocyanine (A1PcS4) at different time intervals after an i.p. injection at a dose of 10 mg kg-1 body weight (b.w.) in tumour and surrounding normal skin and muscle of female C3D2/F1 mice bearing CaD2 mammary carcinoma. Moreover, the photodynamic effect on the tumour and normal skin using sulphonated aluminium phthalocyanines (A1PcS1, A1PcS2, A1pcS4) and Photofrin was compared with respect to dye, dye dose and time interval between dye administration and light exposure. The maximal concentrations of A1PcS2 in the tumour tissue were reached 2-24 h after injection of the dye, while the amounts of A1PcS4 peaked 1-2 h after the dye administration. A1PcS2 was simultaneously localised in the interstitium and in the neoplastic cells of the tumour, whereas A1PcS4 appeared to localise only in the stroma of the tumour. The photodynamic efficiency (light was applied 24 h after dye injection at a dose of 10 mg kg-1 b.w.) of the tumours was found to decrease in the following order: A1PcS2 > A1PcS4 > Photofrin > A1PcS1. Furthermore, photodynamic efficacy was strongly dependent upon dye doses and time intervals between dye administration and light exposure: the higher the dose, the higher the photodynamic efficiency. The most efficient photodynamic therapy (PDT) of the tumour was reached (day 20 tumour-free) when light exposure took place 2 h after injection of A1PcS2 (10 mg kg-1). A dual intratumoral localisation pattern of the dye, as found for A1PcS2, seems desirable to obtain a high photodynamic efficiency. The kinetic patterns of uptake, retention and localisation of A1PcS2 and A1PcS4 are roughly correlated with their photodynamic effect on the tumour and normal skin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellnier D. A., Ho Y. K., Pandey R. K., Missert J. R., Dougherty T. J. Distribution and elimination of Photofrin II in mice. Photochem Photobiol. 1989 Aug;50(2):221–228. doi: 10.1111/j.1751-1097.1989.tb04152.x. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Rosenthal I. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):145–147. doi: 10.1080/09553008514550211. [DOI] [PubMed] [Google Scholar]
- Berg K., Bommer J. C., Moan J. Evaluation of sulfonated aluminum phthalocyanines for use in photochemotherapy. Cellular uptake studies. Cancer Lett. 1989 Jan;44(1):7–15. doi: 10.1016/0304-3835(89)90101-8. [DOI] [PubMed] [Google Scholar]
- Berg K., Bommer J. C., Winkelman J. W., Moan J. Cellular uptake and relative efficiency in cell inactivation by photoactivated sulfonated meso-tetraphenylporphines. Photochem Photobiol. 1990 Oct;52(4):775–781. doi: 10.1111/j.1751-1097.1990.tb08681.x. [DOI] [PubMed] [Google Scholar]
- Boyle R. W., Paquette B., van Lier J. E. Biological activities of phthalocyanines. XIV. Effect of hydrophobic phthalimidomethyl groups on the in vivo phototoxicity and mechanism of photodynamic action of sulphonated aluminium phthalocyanines. Br J Cancer. 1992 Jun;65(6):813–817. doi: 10.1038/bjc.1992.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur N., Ali H., Langlois R., van Lier J. E. Biological activities of phthalocyanines--IX. Photosensitization of V-79 Chinese hamster cells and EMT-6 mouse mammary tumor by selectively sulfonated zinc phthalocyanines. Photochem Photobiol. 1988 May;47(5):705–711. doi: 10.1111/j.1751-1097.1988.tb02768.x. [DOI] [PubMed] [Google Scholar]
- Bremner J. C., Adams G. E., Pearson J. K., Sansom J. M., Stratford I. J., Bedwell J., Bown S. G., MacRobert A. J., Phillips D. Increasing the effect of photodynamic therapy on the RIF-1 murine sarcoma, using the bioreductive drugs RSU1069 and RB6145. Br J Cancer. 1992 Dec;66(6):1070–1076. doi: 10.1038/bjc.1992.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Canti G., Franco P., Marelli O., Cubeddu R., Taroni P., Ramponi R. Comparative study of the therapeutic effect of photoactivated hematoporphyrin derivative and aluminum disulfonated phthalocyanines on tumor bearing mice. Cancer Lett. 1990 Sep;53(2-3):123–127. doi: 10.1016/0304-3835(90)90204-b. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Marshall J. F., Lam G. Y., Hart I. R. Tissue uptake, distribution, and potency of the photoactivatable dye chloroaluminum sulfonated phthalocyanine in mice bearing transplantable tumors. Cancer Res. 1988 Jun 1;48(11):3040–3044. [PubMed] [Google Scholar]
- Chan W. S., Marshall J. F., Svensen R., Bedwell J., Hart I. R. Effect of sulfonation on the cell and tissue distribution of the photosensitizer aluminum phthalocyanine. Cancer Res. 1990 Aug 1;50(15):4533–4538. [PubMed] [Google Scholar]
- Chan W. S., West C. M., Moore J. V., Hart I. R. Photocytotoxic efficacy of sulphonated species of aluminium phthalocyanine against cell monolayers, multicellular spheroids and in vivo tumours. Br J Cancer. 1991 Nov;64(5):827–832. doi: 10.1038/bjc.1991.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Cooper M. T., Mang T. S. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med. 1990;10(5):485–488. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- Evensen J. F., Moan J. A test of different photosensitizers for photodynamic treatment of cancer in a murine tumor model. Photochem Photobiol. 1987 Nov;46(5):859–865. doi: 10.1111/j.1751-1097.1987.tb04860.x. [DOI] [PubMed] [Google Scholar]
- Evensen J. F., Moan J., Winkelman J. W. Toxic and phototoxic effects of tetraphenylporphinesulphonate and haematoporphyrin derivative in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Mar;51(3):477–491. doi: 10.1080/09553008714550961. [DOI] [PubMed] [Google Scholar]
- Ferraudi G., Argüello G. A., Ali H., van Lier J. E. Types I and II sensitized photooxidation of aminoacid by phthalocyanines: a flash photochemical study. Photochem Photobiol. 1988 May;47(5):657–660. doi: 10.1111/j.1751-1097.1988.tb02761.x. [DOI] [PubMed] [Google Scholar]
- Gal D., MacDonald P. C., Porter J. C., Simpson E. R. Cholesterol metabolism in cancer cells in monolayer culture. III. Low-density lipoprotein metabolism. Int J Cancer. 1981 Sep 15;28(3):315–319. doi: 10.1002/ijc.2910280310. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Dougherty T. J. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979 Jan;39(1):146–151. [PubMed] [Google Scholar]
- Hynds S. A., Welsh J., Stewart J. M., Jack A., Soukop M., McArdle C. S., Calman K. C., Packard C. J., Shepherd J. Low-density lipoprotein metabolism in mice with soft tissue tumours. Biochim Biophys Acta. 1984 Oct 4;795(3):589–595. doi: 10.1016/0005-2760(84)90189-9. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989 Apr 19;81(8):570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- Kessel D., Thompson P., Saatio K., Nantwi K. D. Tumor localization and photosensitization by sulfonated derivatives of tetraphenylporphine. Photochem Photobiol. 1987 Jun;45(6):787–790. doi: 10.1111/j.1751-1097.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Kessel D., Woodburn K. Biodistribution of photosensitizing agents. Int J Biochem. 1993 Oct;25(10):1377–1383. doi: 10.1016/0020-711x(93)90685-8. [DOI] [PubMed] [Google Scholar]
- Kimel S., Tromberg B. J., Roberts W. G., Berns M. W. Singlet oxygen generation of porphyrins, chlorins, and phthalocyanines. Photochem Photobiol. 1989 Aug;50(2):175–183. doi: 10.1111/j.1751-1097.1989.tb04145.x. [DOI] [PubMed] [Google Scholar]
- Kongshaug M. Distribution of tetrapyrrole photosensitizers among human plasma proteins. Int J Biochem. 1992 Aug;24(8):1239–1265. doi: 10.1016/0020-711x(92)90200-k. [DOI] [PubMed] [Google Scholar]
- Kongshaug M., Moan J., Brown S. B. The distribution of porphyrins with different tumour localising ability among human plasma proteins. Br J Cancer. 1989 Feb;59(2):184–188. doi: 10.1038/bjc.1989.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshaug M., Rimington C., Evensen J. F., Peng Q., Moan J. Hematoporphyrin diethers--V. Plasma protein binding and photosensitizing efficiency. Int J Biochem. 1990;22(10):1127–1131. doi: 10.1016/0020-711x(90)90110-o. [DOI] [PubMed] [Google Scholar]
- Lombardi P., Norata G., Maggi F. M., Canti G., Franco P., Nicolin A., Catapano A. L. Assimilation of LDL by experimental tumours in mice. Biochim Biophys Acta. 1989 Jun 28;1003(3):301–306. doi: 10.1016/0005-2760(89)90236-1. [DOI] [PubMed] [Google Scholar]
- Moan J., Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991 Apr;53(4):549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Moan J., Peng Q., Evensen J. F., Berg K., Western A., Rimington C. Photosensitizing efficiencies, tumor- and cellular uptake of different photosensitizing drugs relevant for photodynamic therapy of cancer. Photochem Photobiol. 1987 Nov;46(5):713–721. doi: 10.1111/j.1751-1097.1987.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Norata G., Canti G., Ricci L., Nicolin A., Trezzi E., Catapano A. L. In vivo assimilation of low density lipoproteins by a fibrosarcoma tumour line in mice. Cancer Lett. 1984 Dec;25(2):203–208. doi: 10.1016/s0304-3835(84)80046-4. [DOI] [PubMed] [Google Scholar]
- Peng Q., Moan J., Farrants G., Danielsen H. E., Rimington C. Localization of potent photosensitizers in human tumor LOX by means of laser scanning microscopy. Cancer Lett. 1991 Jun 14;58(1-2):17–27. doi: 10.1016/0304-3835(91)90019-e. [DOI] [PubMed] [Google Scholar]
- Peng Q., Moan J., Kongshaug M., Evensen J. F., Anholt H., Rimington C. Sensitizer for photodynamic therapy of cancer: a comparison of the tissue distribution of Photofrin II and aluminum phthalocyanine tetrasulfonate in nude mice bearing a human malignant tumor. Int J Cancer. 1991 May 10;48(2):258–264. doi: 10.1002/ijc.2910480218. [DOI] [PubMed] [Google Scholar]
- Peng Q., Moan J., Nesland J. M., Rimington C. Aluminum phthalocyanines with asymmetrical lower sulfonation and with symmetrical higher sulfonation: a comparison of localizing and photosensitizing mechanism in human tumor LOX xenografts. Int J Cancer. 1990 Oct 15;46(4):719–726. doi: 10.1002/ijc.2910460428. [DOI] [PubMed] [Google Scholar]
- Peng Q., Nesland J. M., Moan J., Evensen J. F., Kongshaug M., Rimington C. Localization of fluorescent Photofrin II and aluminum phthalocyanine tetrasulfonate in transplanted human malignant tumor LOX and normal tissues of nude mice using highly light-sensitive video intensification microscopy. Int J Cancer. 1990 May 15;45(5):972–979. doi: 10.1002/ijc.2910450533. [DOI] [PubMed] [Google Scholar]
- Perry R. R., Smith P. D., Evans S., Pass H. I. Intravenous vs intraperitoneal sensitizer: implications for intraperitoneal photodynamic therapy. Photochem Photobiol. 1991 Mar;53(3):335–340. doi: 10.1111/j.1751-1097.1991.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Qian P., Evensen J. F., Rimington C., Moan J. A comparison of different photosensitizing dyes with respect to uptake C3H-tumors and tissues of mice. Cancer Lett. 1987 Jul;36(1):1–10. doi: 10.1016/0304-3835(87)90096-6. [DOI] [PubMed] [Google Scholar]
- Razum N., Balchum O. J., Profio A. E., Carstens F. Skin photosensitivity: duration and intensity following intravenous hematoporphyrin derivates, HpD and DHE. Photochem Photobiol. 1987 Nov;46(5):925–928. doi: 10.1111/j.1751-1097.1987.tb04870.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem Photobiol. 1991 Jun;53(6):859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Spikes J. D. Phthalocyanines as photosensitizers in biological systems and for the photodynamic therapy of tumors. Photochem Photobiol. 1986 Jun;43(6):691–699. doi: 10.1111/j.1751-1097.1986.tb05648.x. [DOI] [PubMed] [Google Scholar]
- Tralau C. J., Young A. R., Walker N. P., Vernon D. I., MacRobert A. J., Brown S. B., Bown S. G. Mouse skin photosensitivity with dihaematoporphyrin ether (DHE) and aluminium sulphonated phthalocyanine (AlSPc): a comparative study. Photochem Photobiol. 1989 Mar;49(3):305–312. doi: 10.1111/j.1751-1097.1989.tb04111.x. [DOI] [PubMed] [Google Scholar]
- Vitols S., Söderberg-Reid K., Masquelier M., Sjöström B., Peterson C. Low density lipoprotein for delivery of a water-insoluble alkylating agent to malignant cells. In vitro and in vivo studies of a drug-lipoprotein complex. Br J Cancer. 1990 Nov;62(5):724–729. doi: 10.1038/bjc.1990.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKELMAN J. The distribution of tetraphenylporphinesulfonate in the tumor-bearing rat. Cancer Res. 1962 Jun;22:589–596. [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Zalar G. L., Poh-Fitzpatrick M., Krohn D. L., Jacobs R., Harber L. C. Induction of drug photosensitization in man after parenteral exposure to hematoporphyrin. Arch Dermatol. 1977 Oct;113(10):1392–1397. [PubMed] [Google Scholar]
- van Leengoed H. L., van der Veen N., Versteeg A. A., Ouellet R., van Lier J. E., Star W. M. In vivo fluorescence kinetics of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochem Photobiol. 1993 Aug;58(2):233–237. doi: 10.1111/j.1751-1097.1993.tb09554.x. [DOI] [PubMed] [Google Scholar]
- van Leengoed H. L., van der Veen N., Versteeg A. A., Ouellet R., van Lier J. E., Star W. M. In vivo photodynamic effects of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochem Photobiol. 1993 Oct;58(4):575–580. doi: 10.1111/j.1751-1097.1993.tb04935.x. [DOI] [PubMed] [Google Scholar]
- van Lier J. E., Spikes J. D. The chemistry, photophysics and photosensitizing properties of phthalocyanines. Ciba Found Symp. 1989;146:17–32. doi: 10.1002/9780470513842.ch3. [DOI] [PubMed] [Google Scholar]