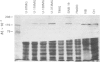
Abstract
We examined levels of mRNA and protein for N-cadherin, the predominant cadherin in neural tissues, and mRNA levels for the cadherin-associated protein, alpha-catenin, in a series of gliomas and in glioblastoma cell lines. mRNA levels for N-cadherin and alpha-catenin were significantly higher in glioblastomas than in low-grade astrocytomas or normal brain, while the levels of intact N-cadherin protein were similar in glioblastomas, low-grade astrocytomas and brain. In addition, there was no consistent relationship between invasiveness and expression of N-cadherin and alpha-catenin in highly invasive vs minimally invasive tumours within the same histopathological grade. To assess further the relationship between cadherin expression and neural tumour invasion, we measured N-cadherin expression, calcium-dependent cell adhesion and motility of several glioblastoma cell lines. While all N-cadherin-expressing lines were adhesive, no correlation was seen between the level of N-cadherin expression and cell motility. Together, these findings imply that, in contrast to the role played by E-cadherin in carcinomas, N-cadherin does not restrict the invasion of glioblastomas.
Full text
PDF

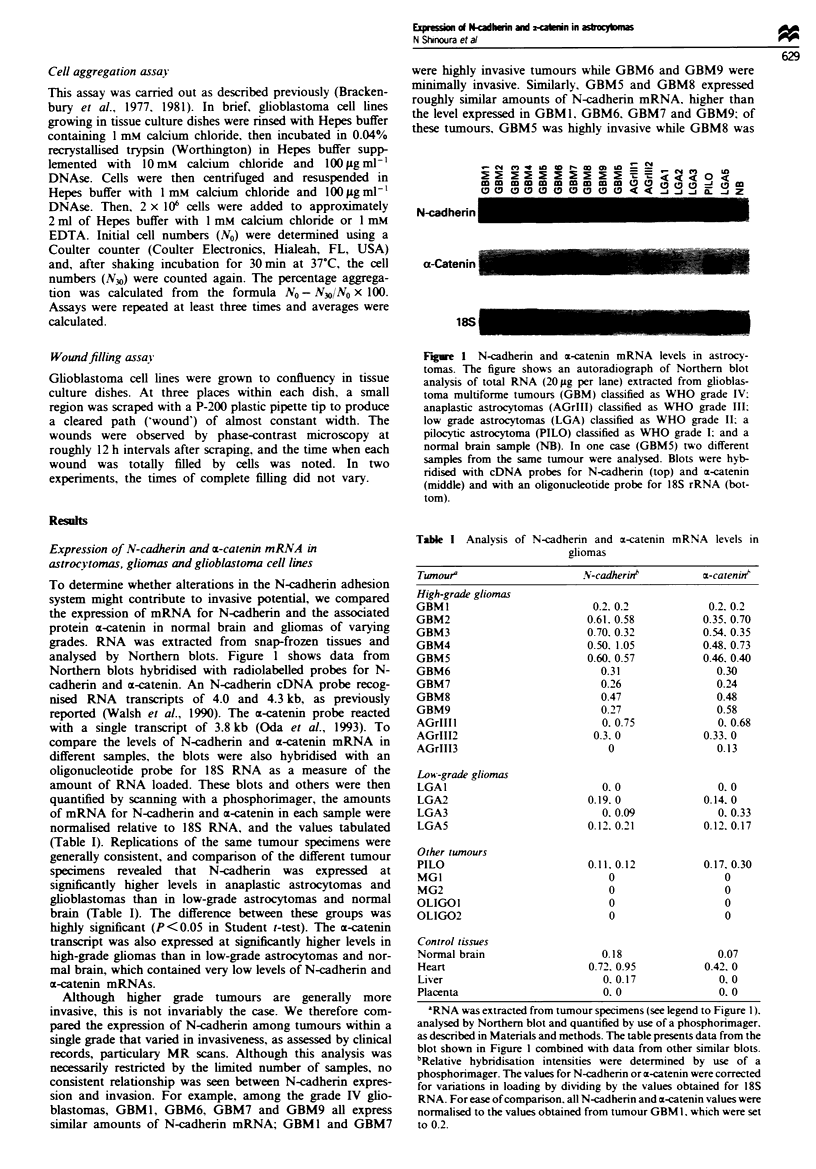
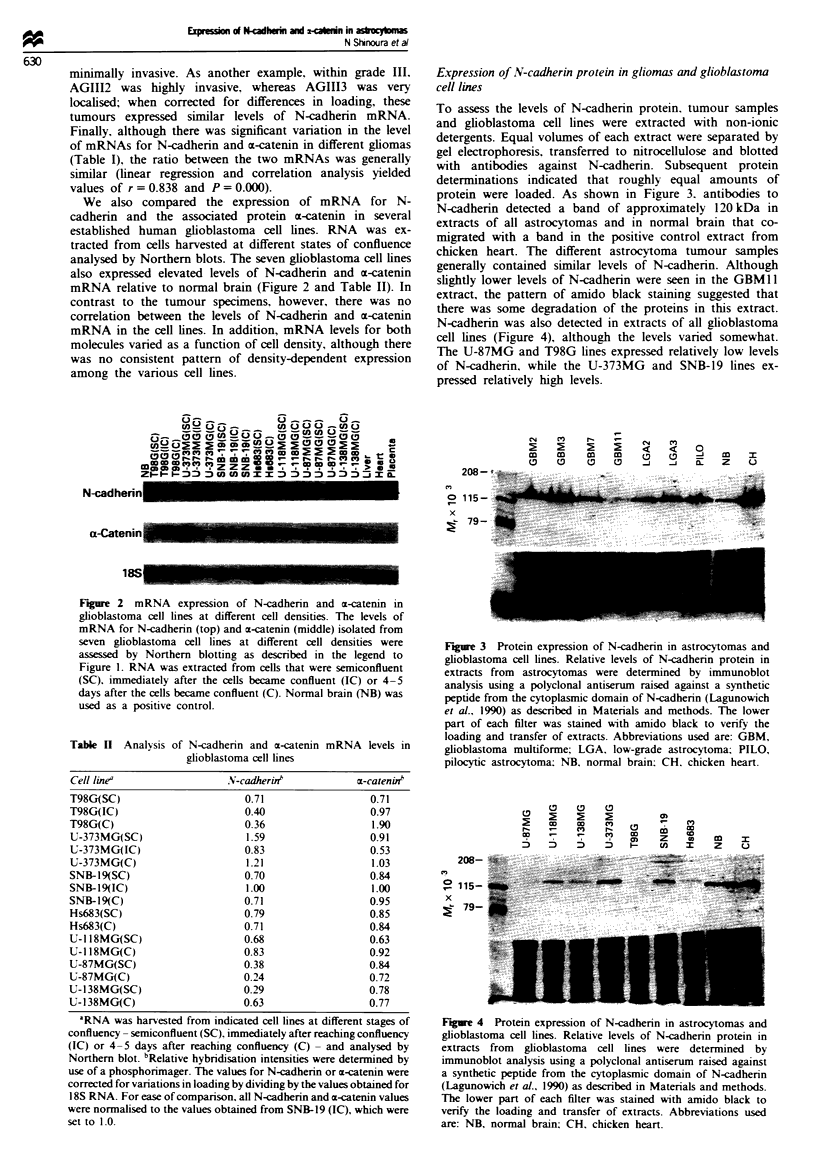



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Rutishauser U., Edelman G. M. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):387–391. doi: 10.1073/pnas.78.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Burger P. C., Heinz E. R., Shibata T., Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988 May;68(5):698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- Chen W. C., Obrink B. Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells. J Cell Biol. 1991 Jul;114(2):319–327. doi: 10.1083/jcb.114.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman D. R. Mechanism of the Invasiveness of Cancer. Science. 1947 Apr 4;105(2727):347–348. doi: 10.1126/science.105.2727.347. [DOI] [PubMed] [Google Scholar]
- Doherty P., Ashton S. V., Moore S. E., Walsh F. S. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991 Oct 4;67(1):21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Dufour S., Hatta K., Takeichi M., Edelman G. M., Thiery J. P. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987 May;104(5):1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen U. H., Behrens J., Sachs M., Eberle G., Voss B., Warda A., Löchner D., Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991 Apr;113(1):173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. L., Behrens D. L., Mullins D. E., Kornblith P. L., Dexter D. L. Plasminogen activator and inhibitor activity in human glioma cells and modulation by sodium butyrate. Cancer Res. 1988 Jan 15;48(2):291–296. [PubMed] [Google Scholar]
- Hatta K., Okada T. S., Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A. 1985 May;82(9):2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Takagi S., Fujisawa H., Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987 Mar;120(1):215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Hatta K., Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986 Apr 3;320(6061):447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hirano S., Kimoto N., Shimoyama Y., Hirohashi S., Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992 Jul 24;70(2):293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Inuzuka H., Miyatani S., Takeichi M. R-cadherin: a novel Ca(2+)-dependent cell-cell adhesion molecule expressed in the retina. Neuron. 1991 Jul;7(1):69–79. doi: 10.1016/0896-6273(91)90075-b. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Shiozaki H., Inoue M., Tamura S., Oka H., Doki Y., Iihara K., Matsui S., Iwazawa T., Nagafuchi A. E-cadherin and alpha-catenin expression in human esophageal cancer. Cancer Res. 1994 Jan 1;54(1):291–296. [PubMed] [Google Scholar]
- Kelly P. J., Daumas-Duport C., Kispert D. B., Kall B. A., Scheithauer B. W., Illig J. J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987 Jun;66(6):865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagunowich L. A., Donoso L. A., Grunwald G. B. Identification of mammalian and invertebrate analogues of the avian calcium-dependent cell adhesion protein N-cadherin with synthetic-peptide directed antibodies against a conserved cytoplasmic domain. Biochem Biophys Res Commun. 1990 Oct 15;172(1):313–320. doi: 10.1016/s0006-291x(05)80211-6. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Mayer B., Johnson J. P., Leitl F., Jauch K. W., Heiss M. M., Schildberg F. W., Birchmeier W., Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993 Apr 1;53(7):1690–1695. [PubMed] [Google Scholar]
- McCallum F. S., Maden B. E. Human 18 S ribosomal RNA sequence inferred from DNA sequence. Variations in 18 S sequences and secondary modification patterns between vertebrates. Biochem J. 1985 Dec 15;232(3):725–733. doi: 10.1042/bj2320725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton R. A., Ewing C. M., Nagafuchi A., Tsukita S., Isaacs W. B. Reduction of E-cadherin levels and deletion of the alpha-catenin gene in human prostate cancer cells. Cancer Res. 1993 Aug 1;53(15):3585–3590. [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano E. W., Venstrom K., Wheeler E. F., Reichardt L. F. Molecular cloning and characterization of B-cadherin, a novel chick cadherin. J Cell Biol. 1991 May;113(4):893–905. doi: 10.1083/jcb.113.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Oda T., Kanai Y., Shimoyama Y., Nagafuchi A., Tsukita S., Hirohashi S. Cloning of the human alpha-catenin cDNA and its aberrant mRNA in a human cancer cell line. Biochem Biophys Res Commun. 1993 Jun 30;193(3):897–904. doi: 10.1006/bbrc.1993.1710. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Nelson-Rees W. A., Springer E. L. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976 Apr;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992 Feb;116(4):989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies N. E., Grunwald G. B. Purification and characterization of NCAD90, a soluble endogenous form of N-cadherin, which is generated by proteolysis during retinal development and retains adhesive and neurite-promoting function. J Neurosci Res. 1993 Sep 1;36(1):33–45. doi: 10.1002/jnr.490360105. [DOI] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Dours-Zimmermann M. T. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991 Sep;7(3):391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- Rasbridge S. A., Gillett C. E., Sampson S. A., Walsh F. S., Millis R. R. Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993 Feb;169(2):245–250. doi: 10.1002/path.1711690211. [DOI] [PubMed] [Google Scholar]
- Reid R. A., Hemperly J. J. Human N-cadherin: nucleotide and deduced amino acid sequence. Nucleic Acids Res. 1990 Oct 11;18(19):5896–5896. doi: 10.1093/nar/18.19.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper J. H., Frixen U. H., Behrens J., Unger A., Jahnke K., Birchmeier W. E-cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res. 1991 Dec 1;51(23 Pt 1):6328–6337. [PubMed] [Google Scholar]
- Shimoyama Y., Nagafuchi A., Fujita S., Gotoh M., Takeichi M., Tsukita S., Hirohashi S. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992 Oct 15;52(20):5770–5774. [PubMed] [Google Scholar]
- Shiozaki H., Tahara H., Oka H., Miyata M., Kobayashi K., Tamura S., Iihara K., Doki Y., Hirano S., Takeichi M. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991 Jul;139(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Okada T. S., Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983 Dec;35(3 Pt 2):631–638. doi: 10.1016/0092-8674(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Sommers C. L., Thompson E. W., Torri J. A., Kemler R., Gelmann E. P., Byers S. W. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ. 1991 Aug;2(8):365–372. [PubMed] [Google Scholar]
- Stein G. H. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J Cell Physiol. 1979 Apr;99(1):43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sano K., Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 1991 Apr;2(4):261–270. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M., Atsumi T., Yoshida C., Uno K., Okada T. S. Selective adhesion of embryonal carcinoma cells and differentiated cells by Ca2+-dependent sites. Dev Biol. 1981 Oct 30;87(2):340–350. doi: 10.1016/0012-1606(81)90157-3. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993 Oct;5(5):806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Neugebauer K. M., Bixby J. L., Lilien J., Reichardt L. F. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988 Mar;1(1):33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet L., Jr, Mareel M., Fiers W., van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991 Jul 12;66(1):107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Walsh F. S., Barton C. H., Putt W., Moore S. E., Kelsell D., Spurr N., Goodfellow P. N. N-cadherin gene maps to human chromosome 18 and is not linked to the E-cadherin gene. J Neurochem. 1990 Sep;55(3):805–812. doi: 10.1111/j.1471-4159.1990.tb04563.x. [DOI] [PubMed] [Google Scholar]