Abstract
The beta-D-galactosidase (beta-gal) gene from Streptococcus thermophilus was cloned to isolate and characterize it for potential use as a selection marker in a food-grade cloning vector. Chromosomal DNA from S. thermophilus 19258 was cleaved with the restriction enzyme PstI and ligated to pBR322 for transformation into Escherichia coli JM108. A beta-galactosidase-positive clone was detected by its blue color on a medium supplemented with 5-bromo-4-chloro-3-indolyl-beta-D-galactoside. This transformant possessed a single plasmid, designated pRH116, which contained, in addition to the vector DNA, a 7.0-kilobase (kb) PstI insertion fragment coding for beta-gal activity. An extract from JM108(pRH116) contained a beta-gal protein with the same electrophoretic mobility as the beta-gal from S. thermophilus 19258. Compared with the beta-gal from E. coli HB101, the S. thermophilus beta-gal was of lower molecular weight. A restriction map of pRH116 was constructed from cleavage of both the plasmid and the purified insert. The construction of deletion derivatives of pRH116 with BglII, BstEII, and HindIII revealed the approximate location of the gene on the 7.0-kb fragment. The beta-gal gene was further localized to a 3.85-kb region.
Full text
PDF

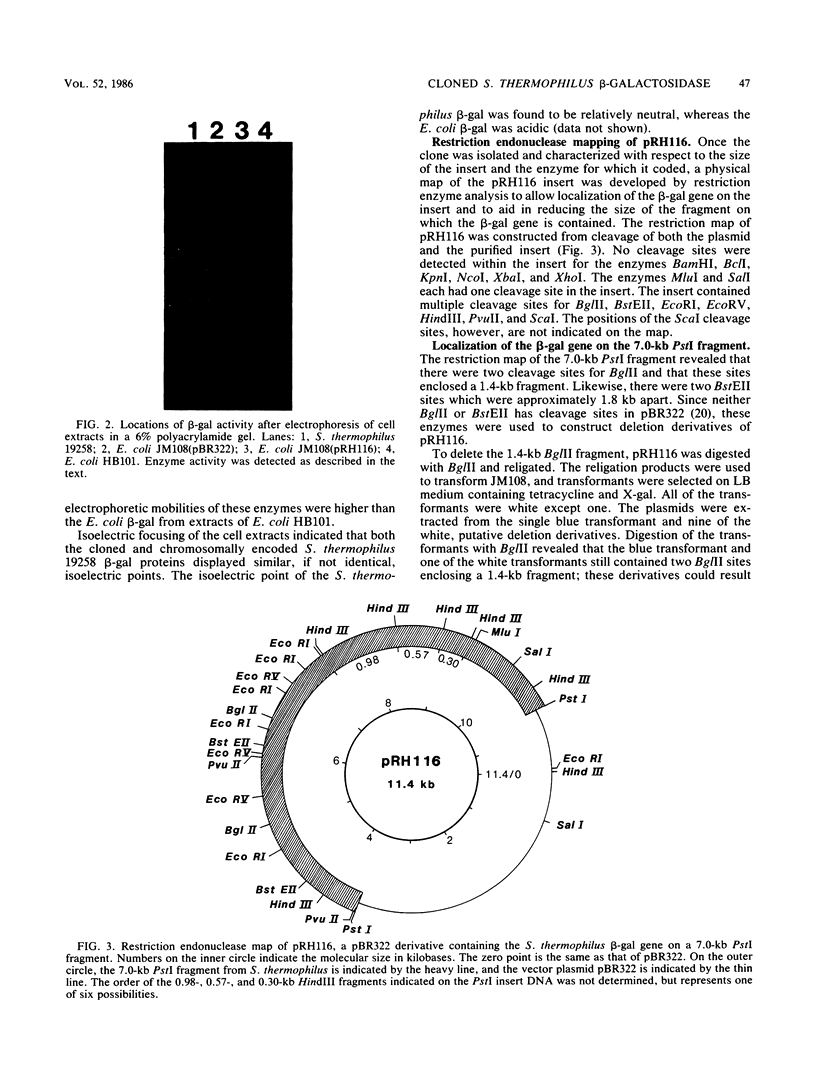



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget P. B., Warner H. R. Identification of P48 and P54 as components of bacteriophage T4 baseplates. J Virol. 1975 Dec;16(6):1669–1677. doi: 10.1128/jvi.16.6.1669-1677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Dao M. L., Ferretti J. J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985 Jan;49(1):115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlander S. K., McKay L. L. Transformation of Streptococcus sanguis Challis with Streptococcus lactis plasmid DNA. Appl Environ Microbiol. 1984 Aug;48(2):342–346. doi: 10.1128/aem.48.2.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. E., McKay L. L. Isolation and partial characterization of plasmid DNA from Streptococcus thermophilus. Appl Environ Microbiol. 1985 Oct;50(4):1103–1106. doi: 10.1128/aem.50.4.1103-1106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutkins R., Morris H. A., McKay L. L. Galactokinase activity in Streptococcus thermophilus. Appl Environ Microbiol. 1985 Oct;50(4):777–780. doi: 10.1128/aem.50.4.777-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. W., Jarvis B. D. Deoxyribonucleic Acid homology among lactic streptococci. Appl Environ Microbiol. 1981 Jan;41(1):77–83. doi: 10.1128/aem.41.1.77-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok J., van Dijl J. M., van der Vossen J. M., Venema G. Cloning and expression of a Streptococcus cremoris proteinase in Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985 Jul;50(1):94–101. doi: 10.1128/aem.50.1.94-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J. K., McKay L. L. Plasmid transformation of Streptococcus lactis protoplasts: optimization and use in molecular cloning. Appl Environ Microbiol. 1984 Aug;48(2):252–259. doi: 10.1128/aem.48.2.252-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Dutta S. M. Lactase activity of microorganisms. Folia Microbiol (Praha) 1978;23(3):210–215. doi: 10.1007/BF02876581. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van der Vossen J. M., Kok J., Venema G. Construction of cloning, promoter-screening, and terminator-screening shuttle vectors for Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985 Aug;50(2):540–542. doi: 10.1128/aem.50.2.540-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]