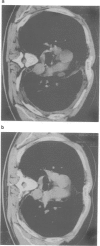
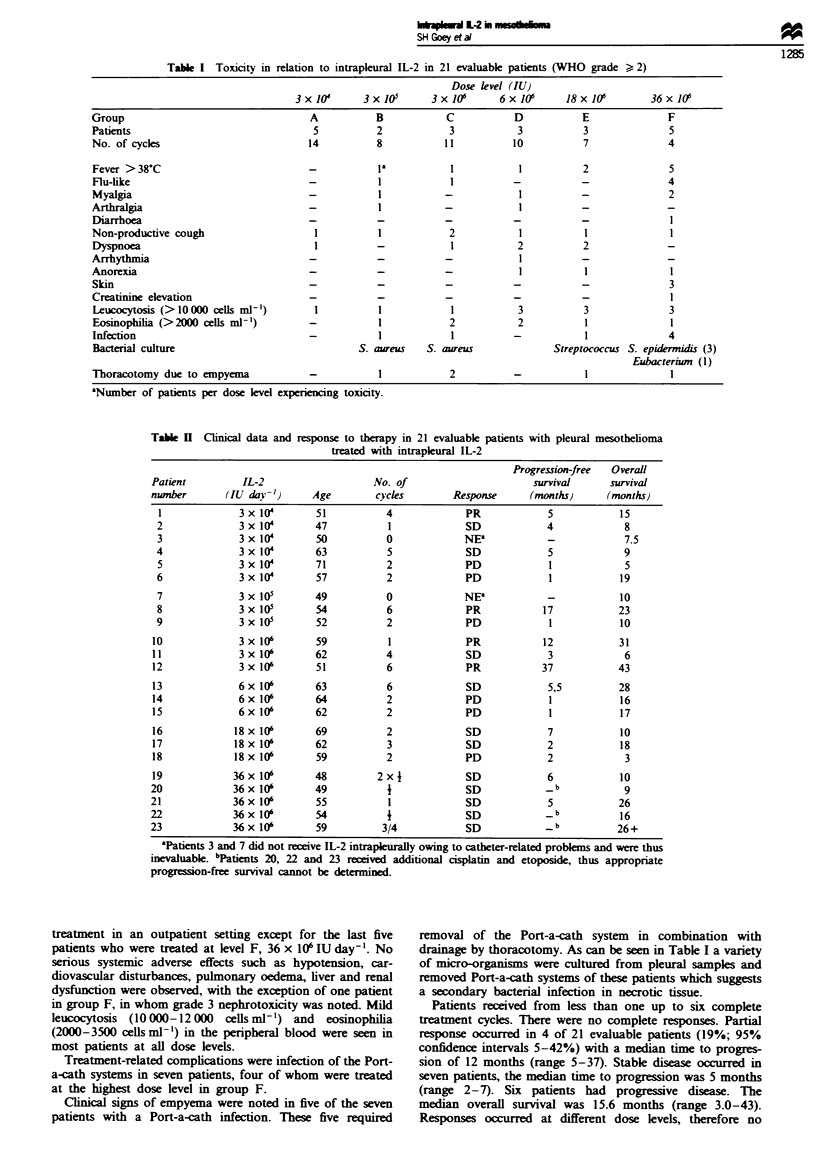
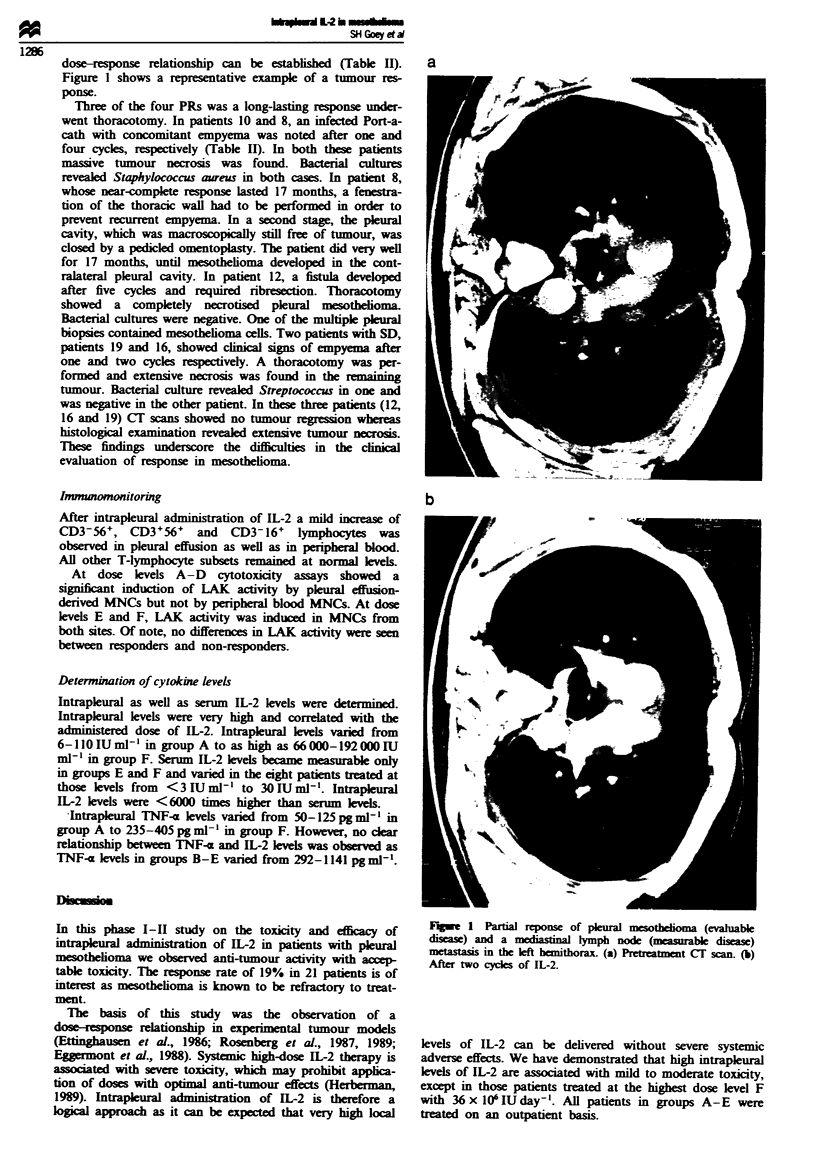
Abstract
Twenty-three patients with pleural mesothelioma stage I-IIA were entered in a study of continuous daily intrapleural infusion of interleukin 2 (IL-2) for 14 days, repeated every 4 weeks. IL-2 was administered according to a groupwise dose escalation schedule (group A, 3 x 10(4); group B, 3 x 10(5); group C, 3 x 10(6); group D, 6 x 10(6); group E, 18 x 10(6); and group F, 36 x 10(6) IU day-1). Each group consisted of at least three patients. Intrapleural administration of IL-2 was associated with acceptable toxicity. All patients were treated on an outpatient basis except for the patients at dose levels E and F. Dose-limiting toxicity was observed at level F, 36 x 10(6) IU daily, and consisted of catheter infection, fever and flu-like symptoms. Intrapleural IL-2 levels were high (> 20,000 IU ml-1) at levels E and F, while serum levels in most patients were not or barely detectable (< 3-30 IU ml-1). Intrapleural IL-2 levels were up to 6000-fold higher than systemic levels. Intrapleural tumour necrosis factor alpha (TNF-alpha) levels varied greatly and did not correlate with IL-2 dosage. Intrapleural mononuclear cells (MNCs) displayed IL-2-induced lymphokine-activated killer (LAK) activity in all patients. Two patients were not evaluable for response owing to catheter-related problems which precluded the delivery of IL-2. Partial response (PR) occurred in 4 of 21 evaluable patients (19%; 95% confidence interval 5-42%) with a median time to progression of 12 months (range 5-37). Stable disease (SD) occurred in seven patients with a median time to progression of 5 months (range 2-7). There were no complete responses (CRs). The median overall survival was 15.6 months (range 3.0-43). No relationship between the dose of IL-2 and response rate was observed. We conclude that IL-2 given intrapleurally is accompanied with acceptable toxicity and has anti-tumour activity against mesothelioma. In view of the refractory nature of the disease IL-2 may be a treatment option for mesothelioma. A formal phase II study is warranted. Based on the observed toxicity, the lack of dose-response relationship and the immunomodulatory effects seen at relatively low-dose IL-2, the recommended dose for a phase II study is 3 x 10(6) IU day-1 using the present treatment schedule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. S., Falkson G., Goedhals L., Vorobiof D. A., Van der Merwe C. A. Malignant pleural mesothelioma: a disease unaffected by current therapeutic maneuvers. J Clin Oncol. 1988 Mar;6(3):527–535. doi: 10.1200/JCO.1988.6.3.527. [DOI] [PubMed] [Google Scholar]
- Astoul P., Viallat J. R., Laurent J. C., Brandely M., Boutin C. Intrapleural recombinant IL-2 in passive immunotherapy for malignant pleural effusion. Chest. 1993 Jan;103(1):209–213. doi: 10.1378/chest.103.1.209. [DOI] [PubMed] [Google Scholar]
- Boutin C., Viallat J. R., Van Zandwijk N., Douillard J. T., Paillard J. C., Guerin J. C., Mignot P., Migueres J., Varlet F., Jehan A. Activity of intrapleural recombinant gamma-interferon in malignant mesothelioma. Cancer. 1991 Apr 15;67(8):2033–2037. doi: 10.1002/1097-0142(19910415)67:8<2033::aid-cncr2820670804>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Butchart E. G., Ashcroft T., Barnsley W. C., Holden M. P. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax. 1976 Feb;31(1):15–24. doi: 10.1136/thx.31.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont A. M., Sugarbaker P. H. Efficacy of chemoimmunotherapy with cyclophosphamide, interleukin-2 and lymphokine activated killer cells in an intraperitoneal murine tumour model. Br J Cancer. 1988 Oct;58(4):410–414. doi: 10.1038/bjc.1988.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinghausen S. E., Rosenberg S. A. Immunotherapy of murine sarcomas using lymphokine activated killer cells: optimization of the schedule and route of administration of recombinant interleukin-2. Cancer Res. 1986 Jun;46(6):2784–2792. [PubMed] [Google Scholar]
- Hillerdal G. Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest. 1983 Oct;77(4):321–343. [PubMed] [Google Scholar]
- Jablons D., Bolton E., Mertins S., Rubin M., Pizzo P., Rosenberg S. A., Lotze M. T. IL-2-based immunotherapy alters circulating neutrophil Fc receptor expression and chemotaxis. J Immunol. 1990 May 1;144(9):3630–3636. [PubMed] [Google Scholar]
- Klempner M. S., Noring R., Mier J. W., Atkins M. B. An acquired chemotactic defect in neutrophils from patients receiving interleukin-2 immunotherapy. N Engl J Med. 1990 Apr 5;322(14):959–965. doi: 10.1056/NEJM199004053221404. [DOI] [PubMed] [Google Scholar]
- Law M. R., Gregor A., Hodson M. E., Bloom H. J., Turner-Warwick M. Malignant mesothelioma of the pleura: a study of 52 treated and 64 untreated patients. Thorax. 1984 Apr;39(4):255–259. doi: 10.1136/thx.39.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning L. S., Bowman R. V., Darby S. B., Robinson B. W. Lysis of human malignant mesothelioma cells by natural killer (NK) and lymphokine-activated killer (LAK) cells. Am Rev Respir Dis. 1989 Jun;139(6):1369–1374. doi: 10.1164/ajrccm/139.6.1369. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Lane H. C., Gallin J. I., Fauci A. S. Marked disparity in incidence of bacterial infections in patients with the acquired immunodeficiency syndrome receiving interleukin-2 or interferon-gamma. Ann Intern Med. 1988 Jan;108(1):36–41. doi: 10.7326/0003-4819-108-1-36. [DOI] [PubMed] [Google Scholar]
- Richards J. M., Gilewski T. A., Vogelzang N. J. Association of interleukin-2 therapy with staphylococcal bacteremia. Cancer. 1991 Mar 15;67(6):1570–1575. doi: 10.1002/1097-0142(19910315)67:6<1570::aid-cncr2820670619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A. The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg. 1988 Aug;208(2):121–135. doi: 10.1097/00000658-198808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso R., Rimoldi R., Salvati F., De Palma M., Cinquegrana A., Nicolò G., Ardizzoni A., Fusco U., Capaccio A., Centofanti R. Intrapleural natural beta interferon in the treatment of malignant pleural effusions. Oncology. 1988;45(3):253–256. doi: 10.1159/000226571. [DOI] [PubMed] [Google Scholar]
- Uchida A., Micksche M., Hoshino T. Intrapleural administration of OK432 in cancer patients: augmentation of autologous tumor killing activity of tumor-associated large granular lymphocytes. Cancer Immunol Immunother. 1984;18(1):5–12. doi: 10.1007/BF00205392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto K., Mivazaki K., Nagashima A., Ishida T., Kuda T., Yano T., Sugimachi K., Nomoto K. Induction of lymphokine-activated killer cells by intrapleural instillations of recombinant interleukin-2 in patients with malignant pleurisy due to lung cancer. Cancer Res. 1987 Apr 15;47(8):2184–2187. [PubMed] [Google Scholar]