Abstract
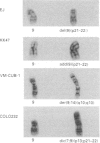
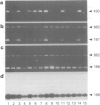
Loss of cell cycle control through the structural or functional aberration of checkpoint genes and their products is a potentially important process in carcinogenesis. In this study, a panel of well-characterised established human bladder cancer cell lines was screened by the polymerase chain reaction for homozygous loss of the cyclin-dependent kinase inhibitor genes p15, p16 and p27. The results demonstrate that, whereas there was no genetic loss of p27, homozygous deletion of both p15 and p16 genes occurred in seven of 13 (54%) independent bladder cell lines tested. Differential loss of either the p15 or p16 gene was not seen. The p15 and p16 genes are known to be juxtaposed on chromosome 9p21 at the locus of a putative tumour-suppressor gene involved in the initiation of bladder cancer. Cytogenetic analysis of the cell lines revealed karyotypes ranging from near diploid to near pentaploid with complex rearrangements of some chromosomes and a high prevalence of chromosome 9p rearrangements, although all cell lines contained at least one cytogenetically normal 9p21 region. These observations support a role for p15/p16 gene inactivation in bladder carcinogenesis and/or the promotion of cell growth in vitro and lend support to the hypothesis that homozygous deletion centred on 9p21 is a mechanism by which both p15 and p16 genes are co-inactivated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonetta L. Tumor-suppressor genes. Open questions on p16. Nature. 1994 Jul 21;370(6486):180–180. doi: 10.1038/370180a0. [DOI] [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cairns P., Shaw M. E., Knowles M. A. Initiation of bladder cancer may involve deletion of a tumour-suppressor gene on chromosome 9. Oncogene. 1993 Apr;8(4):1083–1085. [PubMed] [Google Scholar]
- Cairns P., Tokino K., Eby Y., Sidransky D. Homozygous deletions of 9p21 in primary human bladder tumors detected by comparative multiplex polymerase chain reaction. Cancer Res. 1994 Mar 15;54(6):1422–1424. [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Kastan M. B. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Hirashima M., Ono T., Nakao M., Nishi H., Kimura A., Nomiyama H., Hamada F., Yoshida M. C., Shimada K. Nucleotide sequence of the third cytokine LD78 gene and mapping of all three LD78 gene loci to human chromosome 17. DNA Seq. 1992;3(4):203–212. doi: 10.3109/10425179209034019. [DOI] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Jen J., Harper J. W., Bigner S. H., Bigner D. D., Papadopoulos N., Markowitz S., Willson J. K., Kinzler K. W., Vogelstein B. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994 Dec 15;54(24):6353–6358. [PubMed] [Google Scholar]
- Junien C., Henry I. Genetics of Wilms' tumor: a blend of aberrant development and genomic imprinting. Kidney Int. 1994 Nov;46(5):1264–1279. doi: 10.1038/ki.1994.394. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kamb A., Liu Q., Harshman K., Tavtigian S., Cordon-Cardo C., Skolnick M. H. Response. Science. 1994 Jul 15;265(5170):416–417. doi: 10.1126/science.265.5170.416. [DOI] [PubMed] [Google Scholar]
- Keen A. J., Knowles M. A. Definition of two regions of deletion on chromosome 9 in carcinoma of the bladder. Oncogene. 1994 Jul;9(7):2083–2088. [PubMed] [Google Scholar]
- Marx J. A challenge to p16 gene as a major tumor suppressor. Science. 1994 Jun 24;264(5167):1846–1846. doi: 10.1126/science.8009205. [DOI] [PubMed] [Google Scholar]
- Marx J. How cells cycle toward cancer. Science. 1994 Jan 21;263(5145):319–321. doi: 10.1126/science.8278804. [DOI] [PubMed] [Google Scholar]
- Masters J. R., Bedford P., Kearney A., Povey S., Franks L. M. Bladder cancer cell line cross-contamination: identification using a locus-specific minisatellite probe. Br J Cancer. 1988 Mar;57(3):284–286. doi: 10.1038/bjc.1988.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters J. R., Hepburn P. J., Walker L., Highman W. J., Trejdosiewicz L. K., Povey S., Parkar M., Hill B. T., Riddle P. R., Franks L. M. Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res. 1986 Jul;46(7):3630–3636. [PubMed] [Google Scholar]
- Miyao N., Tsai Y. C., Lerner S. P., Olumi A. F., Spruck C. H., 3rd, Gonzalez-Zulueta M., Nichols P. W., Skinner D. G., Jones P. A. Role of chromosome 9 in human bladder cancer. Cancer Res. 1993 Sep 1;53(17):4066–4070. [PubMed] [Google Scholar]
- Niell H. B., Soloway M. S. Use of the tumour colony assay in the evaluation of patients with bladder cancer. Br J Urol. 1983 Jun;55(3):271–274. doi: 10.1111/j.1464-410x.1983.tb03296.x. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- O'Toole C. M., Povey S., Hepburn P., Franks L. M. Identity of some human bladder cancer cell lines. Nature. 1983 Feb 3;301(5899):429–430. doi: 10.1038/301429a0. [DOI] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Ruppert J. M., Tokino K., Sidransky D. Evidence for two bladder cancer suppressor loci on human chromosome 9. Cancer Res. 1993 Nov 1;53(21):5093–5095. [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Von Eschenbach A., Tsai Y. C., Jones P., Summerhayes I., Marshall F., Paul M., Green P., Hamilton S. R., Frost P. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991 May 3;252(5006):706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Southgate J., Hutton K. A., Thomas D. F., Trejdosiewicz L. K. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest. 1994 Oct;71(4):583–594. [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Stadler W. M., Sherman J., Bohlander S. K., Roulston D., Dreyling M., Rukstalis D., Olopade O. I. Homozygous deletions within chromosomal bands 9p21-22 in bladder cancer. Cancer Res. 1994 Apr 15;54(8):2060–2063. [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Southgate J., Donald J. A., Masters J. R., Hepburn P. J., Hodges G. M. Monoclonal antibodies to human urothelial cell lines and hybrids: production and characterization. J Urol. 1985 Mar;133(3):533–538. doi: 10.1016/s0022-5347(17)49048-3. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993 Aug;7(8):1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- Yeager T., Stadler W., Belair C., Puthenveettil J., Olopade O., Reznikoff C. Increased p16 levels correlate with pRb alterations in human urothelial cells. Cancer Res. 1995 Feb 1;55(3):493–497. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]