Abstract
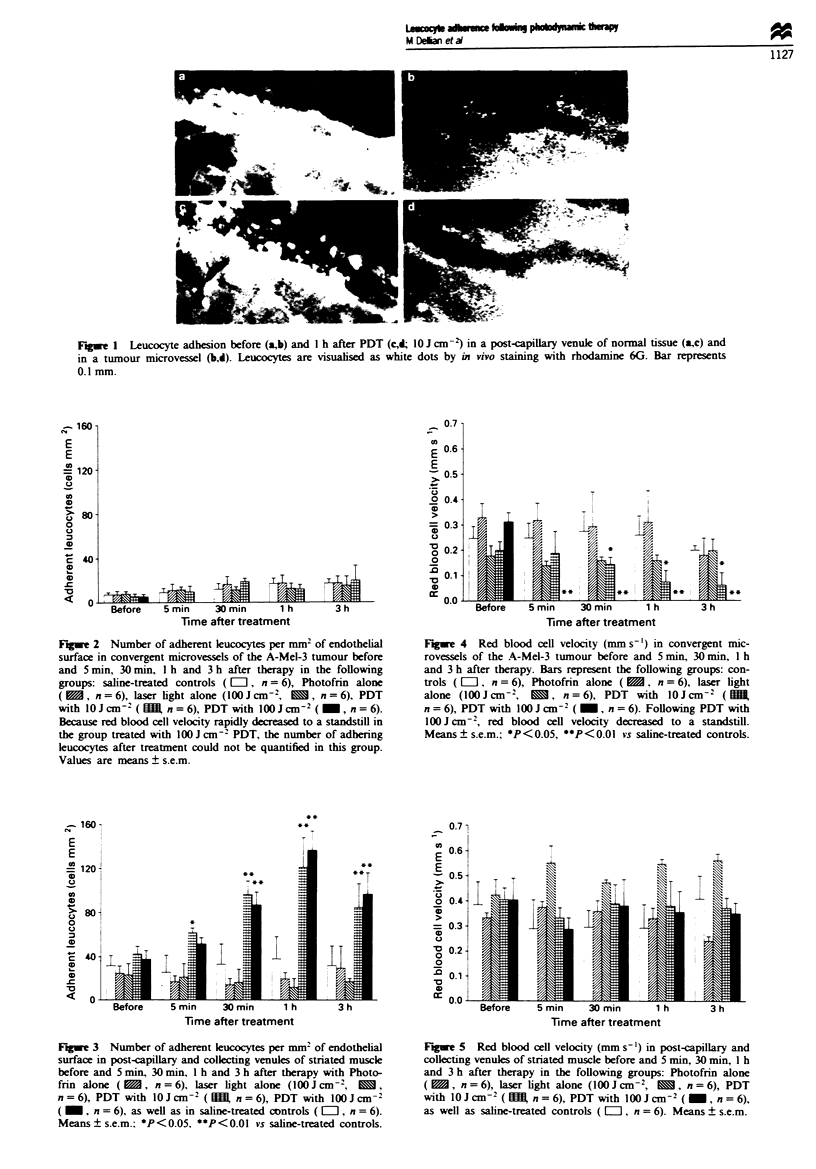
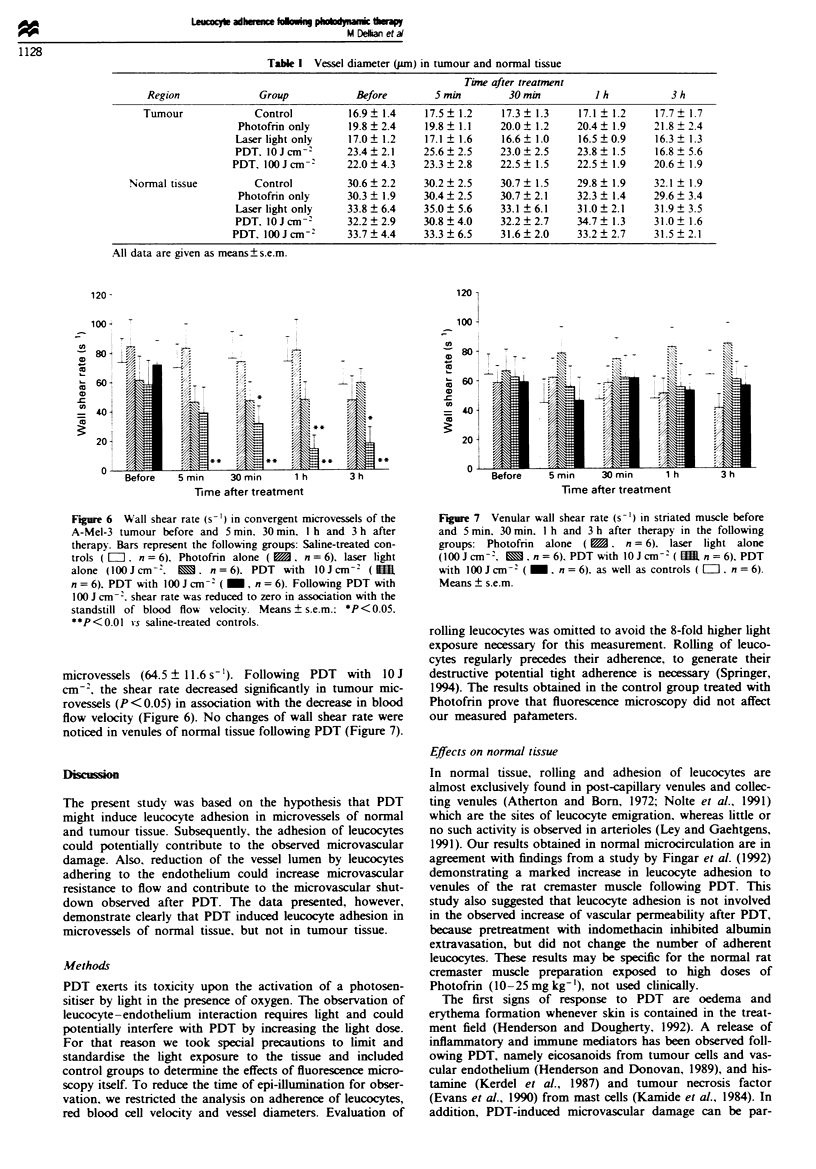
An inflammatory reaction is regularly noticed in irradiated tissues following photodynamic therapy (PDT). This observation is potentially associated with leucocyte-mediated tissue damage, which might further contribute to the tumoricidal effect of this therapy. The objective of our study was to investigate the effects of PDT on leucocyte-endothelium interaction in the microvasculature of tumours and normal tissue. Experiments were performed in the dorsal skinfold chamber preparation of Syrian golden hamsters bearing amelanotic melanoma A-Mel-3. The photosensitiser. Photofrin (5 mg kg-1 i.v.) was injected 24 h before laser irradiation (630 nm, 100 mW cm-2, 10 J cm-2 or 100 J cm-2). Post-capillary confluent venules (diameter 15-40 microns) of subcutaneous (s.c.) tissue or the amelanotic melanoma A-Mel-3 were observed by intravital microscopy before, 5, 30, 60 and 180 min after laser irradiation and recorded for off-line analysis. Before treatment, the number of adherent leucocytes in tumour vessels was only 22% of the number observed in vessels of s.c. tissue (P < 0.01). The maximum increase in adhering leucocytes was observed in post-capillary venules of s.c. tissue 1 h after PDT (P < 0.01). In contrast, enhanced leucocyte-endothelium interaction was missing in tumour vessels and in control groups. These results indicate that the tumour destruction observed after PDT is not mediated by leucocyte-endothelium interaction in the tumour. Induction of leucocyte adhesion in the PDT-treated normal tissue suggests a contribution to the peritumoral inflammatory response. Different maturational status or biochemical properties of tumour microvascular endothelium may explain the lack of leucocyte adherence upon PDT.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaishi K., Endrich B., Götz A., Messmer K. Quantitative analysis of microvascular structure and function in the amelanotic melanoma A-Mel-3. Cancer Res. 1981 May;41(5):1898–1904. [PubMed] [Google Scholar]
- Athar M., Elmets C. A., Bickers D. R., Mukhtar H. A novel mechanism for the generation of superoxide anions in hematoporphyrin derivative-mediated cutaneous photosensitization. Activation of the xanthine oxidase pathway. J Clin Invest. 1989 Apr;83(4):1137–1143. doi: 10.1172/JCI113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Cooper M. T., Mang T. S. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med. 1990;10(5):485–488. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- Endrich B., Asaishi K., Götz A., Messmer K. Technical report--a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 1980;177(2):125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- Evans S., Matthews W., Perry R., Fraker D., Norton J., Pass H. I. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990 Jan 3;82(1):34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- FORTNER J. G., MAHY A. G., SCHRODT G. R. Transplantable tumors of the Syrian (golden) hamster. I. Tumors of the alimentary tract, endocrine glands and melanomas. Cancer Res. 1961 Jul;21(6):161–198. [PubMed] [Google Scholar]
- Fingar V. H., Henderson B. W. Drug and light dose dependence of photodynamic therapy: a study of tumor and normal tissue response. Photochem Photobiol. 1987 Nov;46(5):837–841. doi: 10.1111/j.1751-1097.1987.tb04856.x. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Siegel K. A., Wieman T. J., Doak K. W. The effects of thromboxane inhibitors on the microvascular and tumor response to photodynamic therapy. Photochem Photobiol. 1993 Sep;58(3):393–399. doi: 10.1111/j.1751-1097.1993.tb09580.x. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Wieman T. J., Wiehle S. A., Cerrito P. B. The role of microvascular damage in photodynamic therapy: the effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992 Sep 15;52(18):4914–4921. [PubMed] [Google Scholar]
- Gomer C. J., Rucker N., Murphree A. L. Differential cell photosensitivity following porphyrin photodynamic therapy. Cancer Res. 1988 Aug 15;48(16):4539–4542. [PubMed] [Google Scholar]
- Henderson B. W., Donovan J. M. Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer Res. 1989 Dec 15;49(24 Pt 1):6896–6900. [PubMed] [Google Scholar]
- Henderson B. W., Dougherty T. J. How does photodynamic therapy work? Photochem Photobiol. 1992 Jan;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- Kamide R., Gigli I., Lim H. W. Participation of mast cells and complement in the immediate phase of hematoporphyrin-induced phototoxicity. J Invest Dermatol. 1984 May;82(5):485–490. doi: 10.1111/1523-1747.ep12261010. [DOI] [PubMed] [Google Scholar]
- Kerdel F. A., Soter N. A., Lim H. W. In vivo mediator release and degranulation of mast cells in hematoporphyrin derivative-induced phototoxicity in mice. J Invest Dermatol. 1987 Mar;88(3):277–280. doi: 10.1111/1523-1747.ep12466135. [DOI] [PubMed] [Google Scholar]
- Kuzu I., Bicknell R., Fletcher C. D., Gatter K. C. Expression of adhesion molecules on the endothelium of normal tissue vessels and vascular tumors. Lab Invest. 1993 Sep;69(3):322–328. [PubMed] [Google Scholar]
- Leunig A., Staub F., Peters J., Heimann A., Csapo C., Kempski O., Goetz A. E. Relation of early Photofrin uptake to photodynamically induced phototoxicity and changes of cell volume in different cell lines. Eur J Cancer. 1994;30A(1):78–83. doi: 10.1016/s0959-8049(05)80023-1. [DOI] [PubMed] [Google Scholar]
- Leunig M., Leunig A., Lankes P., Goetz A. E. Evaluation of photodynamic therapy-induced heating of hamster melanoma and its effect on local tumour eradication. Int J Hyperthermia. 1994 Mar-Apr;10(2):297–306. doi: 10.3109/02656739409009350. [DOI] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991 Oct;69(4):1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- Lim H. W., Young L., Hagan M., Gigli I. Delayed phase of hematoporphyrin-induced phototoxicity: modulation by complement, leukocytes, and antihistamines. J Invest Dermatol. 1985 Feb;84(2):114–117. doi: 10.1111/1523-1747.ep12275345. [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Zweifach B. W. Application of the "two-slit" photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978 Jan;15(1):93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- Nolte D., Lehr H. A., Messmer K. Adenosine inhibits postischemic leukocyte-endothelium interaction in postcapillary venules of the hamster. Am J Physiol. 1991 Sep;261(3 Pt 2):H651–H655. doi: 10.1152/ajpheart.1991.261.3.H651. [DOI] [PubMed] [Google Scholar]
- Ohkubo C., Bigos D., Jain R. K. Interleukin 2 induced leukocyte adhesion to the normal and tumor microvascular endothelium in vivo and its inhibition by dextran sulfate: implications for vascular leak syndrome. Cancer Res. 1991 Mar 1;51(5):1561–1563. [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McEver R. P., McIntyre T. M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991 Feb;112(4):749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. W., Schuschke D. A., Miller F. N. Prostanoid antagonists inhibit the response of the microcirculation to "early" photodynamic therapy. Radiat Res. 1991 Sep;127(3):292–296. [PubMed] [Google Scholar]
- Reed M. W., Wieman T. J., Schuschke D. A., Tseng M. T., Miller F. N. A comparison of the effects of photodynamic therapy on normal and tumor blood vessels in the rat microcirculation. Radiat Res. 1989 Sep;119(3):542–552. [PubMed] [Google Scholar]
- Renard N., Liénard D., Lespagnard L., Eggermont A., Heimann R., Lejeune F. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha). Int J Cancer. 1994 Jun 1;57(5):656–663. doi: 10.1002/ijc.2910570508. [DOI] [PubMed] [Google Scholar]
- Shumaker B. P., Hetzel F. W. Clinical laser photodynamic therapy in the treatment of bladder carcinoma. Photochem Photobiol. 1987 Nov;46(5):899–901. doi: 10.1111/j.1751-1097.1987.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E. Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput Methods Programs Biomed. 1986 Aug;23(1):57–62. doi: 10.1016/0169-2607(86)90081-7. [DOI] [PubMed] [Google Scholar]
- Wu N. Z., Klitzman B., Dodge R., Dewhirst M. W. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992 Aug 1;52(15):4265–4268. [PubMed] [Google Scholar]
- Wu N. Z., Ross B. A., Gulledge C., Klitzman B., Dodge R., Dewhirst M. W. Differences in leucocyte-endothelium interactions between normal and adenocarcinoma bearing tissues in response to radiation. Br J Cancer. 1994 May;69(5):883–889. doi: 10.1038/bjc.1994.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerhackl B., Parekh N., Brinkhus H., Steinhausen M. The use of fluorescent labeled erythrocytes for intravital investigation of flow and local hematocrit in glomerular capillaries in the rat. Int J Microcirc Clin Exp. 1983;2(2):119–129. [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]




