Abstract
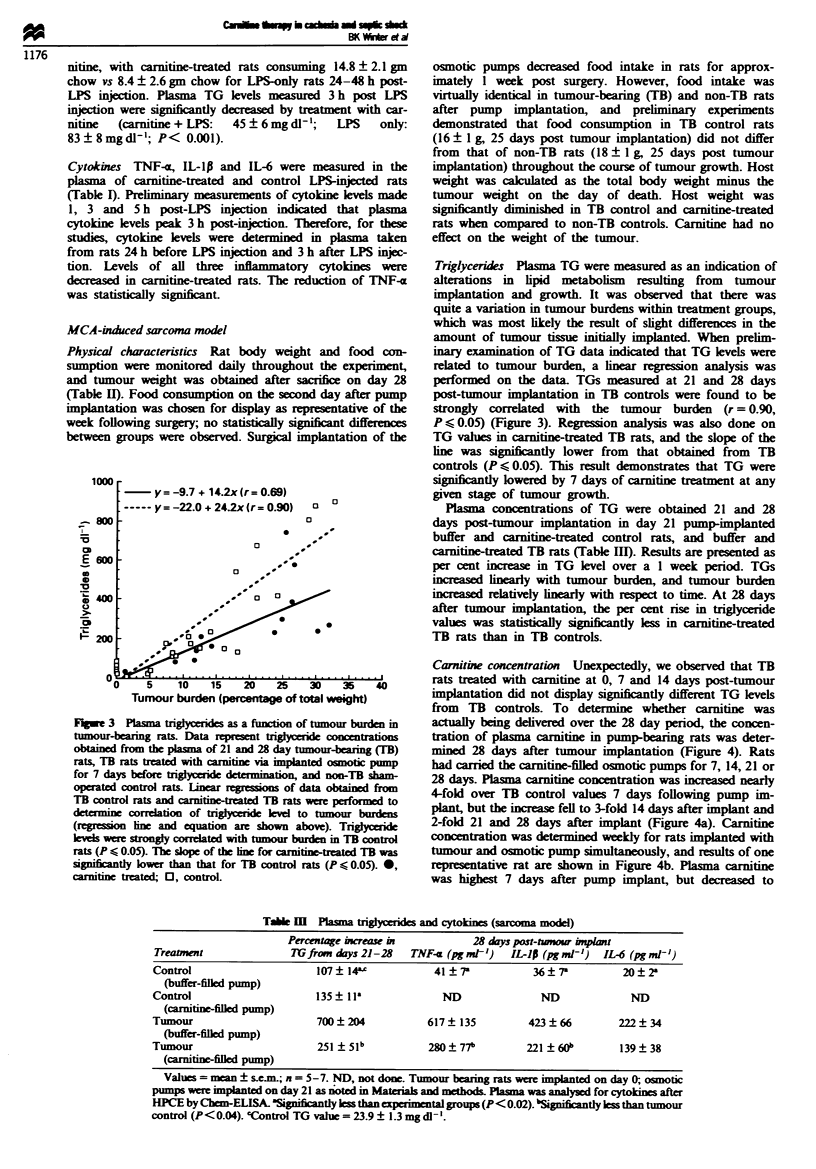
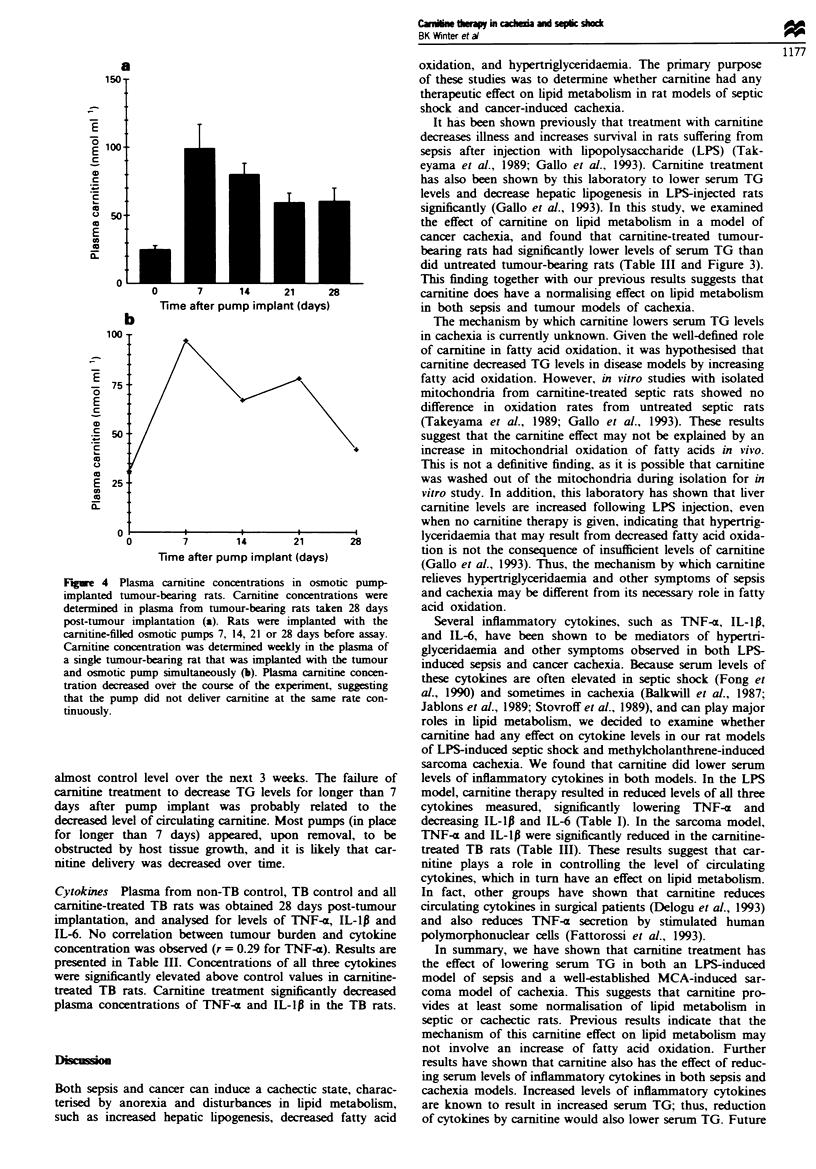
Inappropriate hepatic lipogenesis, hypertriglyceridaemia, decreased fatty acid oxidation and muscle protein wasting are common in patients with sepsis, cancer or AIDS. Given carnitine's role in the oxidation of fatty acids (FAs), we anticipated that carnitine might promote FA oxidation, thus ameliorating metabolic disturbances in lipopolysaccharide (LPS)- and methylcholanthrene-induced sarcoma models of wasting in rats. In the LPS model, rats were injected with LPS (24 mg kg-1 i.p.), and treated with carnitine (100 mg kg-1 i.p.) at -16, -8, 0 and 8 h post LPS. Rat health was observed, and plasma inflammatory cytokines and triglycerides (TG) were measured before and 3 h post LPS. In the sarcoma model, rats were implanted subcutaneously with tumour, and treated continuously with carnitine (200 mg kg-1 day-1 i.p.) via implanted osmotic pumps. Tumour burden, TG and cytokines were measured weekly for 4 weeks. Carnitine treatment significantly lowered the tumour-induced rise in TG (% rise) in the sarcoma model (700 +/- 204 vs 251 +/- 51, P < 0.03) in control and carnitine groups respectively. Levels of interleukin-1 beta (IL-1 beta), interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-alpha) (pg ml-1) were also lowered by carnitine in both LPS (IL-1 beta: 536 +/- 65 vs 378 +/- 44: IL-6: 271 +/- 29 vs 222 +/- 32; TNF-alpha: 618 +/- 86 vs 367 +/- 54, P < or = 0.02) and sarcoma models (IL-1 beta: 423 +/- 33 vs 221 +/- 60; IL-6: 222 +/- 18 vs 139 +/- 38; TNF-alpha: 617 +/- 69 vs 280 +/- 77, P < or = 0.05) for control and carnitine groups respectively. We conclude that carnitine has a therapeutic effect on morbidity and lipid metabolism in these disease models, and that these effects could be the result of down-regulation of cytokine production and/or increased clearance of cytokines.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias-Díaz J., Vara E., Gómez M., Moreno A., Torres-Melero J., Balibrea J. L. Effect of sepsis-related cytokines on lipid synthesis by isolated human hepatocytes. Eur J Surg. 1993 Oct;159(10):535–539. [PubMed] [Google Scholar]
- Balkwill F., Osborne R., Burke F., Naylor S., Talbot D., Durbin H., Tavernier J., Fiers W. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet. 1987 Nov 28;2(8570):1229–1232. doi: 10.1016/s0140-6736(87)91850-2. [DOI] [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Effect of megestrol acetate on weight loss induced by tumour necrosis factor alpha and a cachexia-inducing tumour (MAC16) in NMRI mice. Br J Cancer. 1990 Sep;62(3):420–424. doi: 10.1038/bjc.1990.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackham M., Cesar D., Park O. J., Vary T. C., Wu K., Kaempfer S., Shackleton C. H., Hellerstein M. K. Effects of recombinant monokines on hepatic pyruvate dehydrogenase, pyruvate dehydrogenase kinase, lipogenesis de novo and plasma triacylglycerols. Abolition by prior fasting. Biochem J. 1992 May 15;284(Pt 1):129–135. doi: 10.1042/bj2840129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Burt M. E., Lowry S. F., Gorschboth C., Brennan M. F. Metabolic alterations in a noncachectic animal tumor system. Cancer. 1981 May 1;47(9):2138–2146. doi: 10.1002/1097-0142(19810501)47:9<2138::aid-cncr2820470906>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Chance W. T., Cao L., Zhang F. S., Fischer J. E. Clenbuterol plus acivicin decrease tumor growth and increase muscle mass in rats maintained on total parenteral nutrition. Am J Surg. 1991 Jan;161(1):51–56. doi: 10.1016/0002-9610(91)90360-p. [DOI] [PubMed] [Google Scholar]
- Darling G., Fraker D. L., Jensen J. C., Gorschboth C. M., Norton J. A. Cachectic effects of recombinant human tumor necrosis factor in rats. Cancer Res. 1990 Jul 1;50(13):4008–4013. [PubMed] [Google Scholar]
- Ettinger W. H., Miller L. A., Smith T. K., Parks J. S. Effect of interleukin-1 alpha on lipoprotein lipids in cynomolgus monkeys: comparison to tumor necrosis factor. Biochim Biophys Acta. 1992 Oct 30;1128(2-3):186–192. doi: 10.1016/0005-2760(92)90306-g. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Adi S., Staprans I., Neese R., Shigenaga J., Doerrler W., Moser A., Dinarello C. A., Grunfeld C. Effect of interleukin-1 on lipid metabolism in the rat. Similarities to and differences from tumor necrosis factor. Arterioscler Thromb. 1991 May-Jun;11(3):495–500. doi: 10.1161/01.atv.11.3.495. [DOI] [PubMed] [Google Scholar]
- Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990 Jun;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong S. T., Mednis A., Remold H. G. Interferon-gamma stimulates lipid metabolism in human monocytes. Cell Immunol. 1992 Aug;143(1):108–117. doi: 10.1016/0008-8749(92)90009-e. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Dinarello C. A., Feingold K. R. Tumor necrosis factor-alpha, interleukin-1, and interferon alpha stimulate triglyceride synthesis in HepG2 cells. Metabolism. 1991 Sep;40(9):894–898. doi: 10.1016/0026-0495(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Feingold K. R. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992 Jul 30;327(5):329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Feingold K. R. The role of the cytokines, interferon alpha and tumor necrosis factor in the hypertriglyceridemia and wasting of AIDs. J Nutr. 1992 Mar;122(3 Suppl):749–753. doi: 10.1093/jn/122.suppl_3.749. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Verdier J. A., Neese R., Moser A. H., Feingold K. R. Mechanisms by which tumor necrosis factor stimulates hepatic fatty acid synthesis in vivo. J Lipid Res. 1988 Oct;29(10):1327–1335. [PubMed] [Google Scholar]
- Jablons D. M., McIntosh J. K., Mulé J. J., Nordan R. P., Rudikoff S., Lotze M. T. Induction of interferon-beta 2/interleukin-6 (IL-6) by cytokine administration and detection of circulating interleukin-6 in the tumor-bearing state. Ann N Y Acad Sci. 1989;557:157–161. doi: 10.1111/j.1749-6632.1989.tb24008.x. [DOI] [PubMed] [Google Scholar]
- Kern K. A., Norton J. A. Cancer cachexia. JPEN J Parenter Enteral Nutr. 1988 May-Jun;12(3):286–298. doi: 10.1177/0148607188012003286. [DOI] [PubMed] [Google Scholar]
- Langstein H. N., Doherty G. M., Fraker D. L., Buresh C. M., Norton J. A. The roles of gamma-interferon and tumor necrosis factor alpha in an experimental rat model of cancer cachexia. Cancer Res. 1991 May 1;51(9):2302–2306. [PubMed] [Google Scholar]
- Langstein H. N., Norton J. A. Mechanisms of cancer cachexia. Hematol Oncol Clin North Am. 1991 Feb;5(1):103–123. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. An improved and simplified radioisotopic assay for the determination of free and esterified carnitine. J Lipid Res. 1976 May;17(3):277–281. [PubMed] [Google Scholar]
- Memon R. A., Grunfeld C., Moser A. H., Feingold K. R. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 1993 May;132(5):2246–2253. doi: 10.1210/endo.132.5.8477669. [DOI] [PubMed] [Google Scholar]
- Moley J. F., Morrison S. D., Norton J. A. Insulin reversal of cancer cachexia in rats. Cancer Res. 1985 Oct;45(10):4925–4931. [PubMed] [Google Scholar]
- Nelson K. A., Walsh D., Sheehan F. A. The cancer anorexia-cachexia syndrome. J Clin Oncol. 1994 Jan;12(1):213–225. doi: 10.1200/JCO.1994.12.1.213. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Vydelingum N. A., Younes R. N., Fried S. K., Brennan M. F. Tumor-induced alterations in tissue lipoprotein lipase activity and mRNA levels. Cancer Res. 1991 Feb 1;51(3):863–869. [PubMed] [Google Scholar]
- Phillips T. M., Kimmel P. L. High-performance capillary electrophoretic analysis of inflammatory cytokines in human biopsies. J Chromatogr B Biomed Appl. 1994 Jun 3;656(1):259–266. doi: 10.1016/0378-4347(94)00036-0. [DOI] [PubMed] [Google Scholar]
- Popp M. B., Morrison S. D., Brennan M. F. Total parenteral nutrition in a methylcholanthrene-induced rat sarcoma model. Cancer Treat Rep. 1981;65 (Suppl 5):137–143. [PubMed] [Google Scholar]
- Sherry B. A., Gelin J., Fong Y., Marano M., Wei H., Cerami A., Lowry S. F., Lundholm K. G., Moldawer L. L. Anticachectin/tumor necrosis factor-alpha antibodies attenuate development of cachexia in tumor models. FASEB J. 1989 Jun;3(8):1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Tisdale M. J. Mechanism of muscle protein degradation in cancer cachexia. Br J Cancer. 1993 Aug;68(2):314–318. doi: 10.1038/bjc.1993.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallion A., Zhang F. S., Chance W. T., Foley-Nelson T., Fischer J. E. Reversal of cancer cachexia in rats by cimaterol and supplemental nutrition. Surgery. 1991 Oct;110(4):678–684. [PubMed] [Google Scholar]
- Stovroff M. C., Fraker D. L., Norton J. A. Cachectin activity in the serum of cachectic, tumor-bearing rats. Arch Surg. 1989 Jan;124(1):94–99. doi: 10.1001/archsurg.1989.01410010104021. [DOI] [PubMed] [Google Scholar]
- Strassmann G., Fong M., Windsor S., Neta R. The role of interleukin-6 in lipopolysaccharide-induced weight loss, hypoglycemia and fibrinogen production, in vivo. Cytokine. 1993 Jul;5(4):285–290. doi: 10.1016/1043-4666(93)90058-d. [DOI] [PubMed] [Google Scholar]
- Takeyama N., Takagi D., Matsuo N., Kitazawa Y., Tanaka T. Altered hepatic fatty acid metabolism in endotoxicosis: effect of L-carnitine on survival. Am J Physiol. 1989 Jan;256(1 Pt 1):E31–E38. doi: 10.1152/ajpendo.1989.256.1.E31. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Von Roenn J. H., Roth E. L., Craig R. HIV-related cachexia: potential mechanisms and treatment. Oncology. 1992;49 (Suppl 2):50–54. doi: 10.1159/000227129. [DOI] [PubMed] [Google Scholar]
- Welbourn C. R., Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg. 1992 Oct;79(10):998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- Worthley L. I., Fishlock R. C., Snoswell A. M. Carnitine deficiency with hyperbilirubinemia, generalized skeletal muscle weakness and reactive hypoglycemia in a patient on long-term total parenteral nutrition: treatment with intravenous L-carnitine. JPEN J Parenter Enteral Nutr. 1983 Mar-Apr;7(2):176–180. doi: 10.1177/0148607183007002176. [DOI] [PubMed] [Google Scholar]


