Abstract
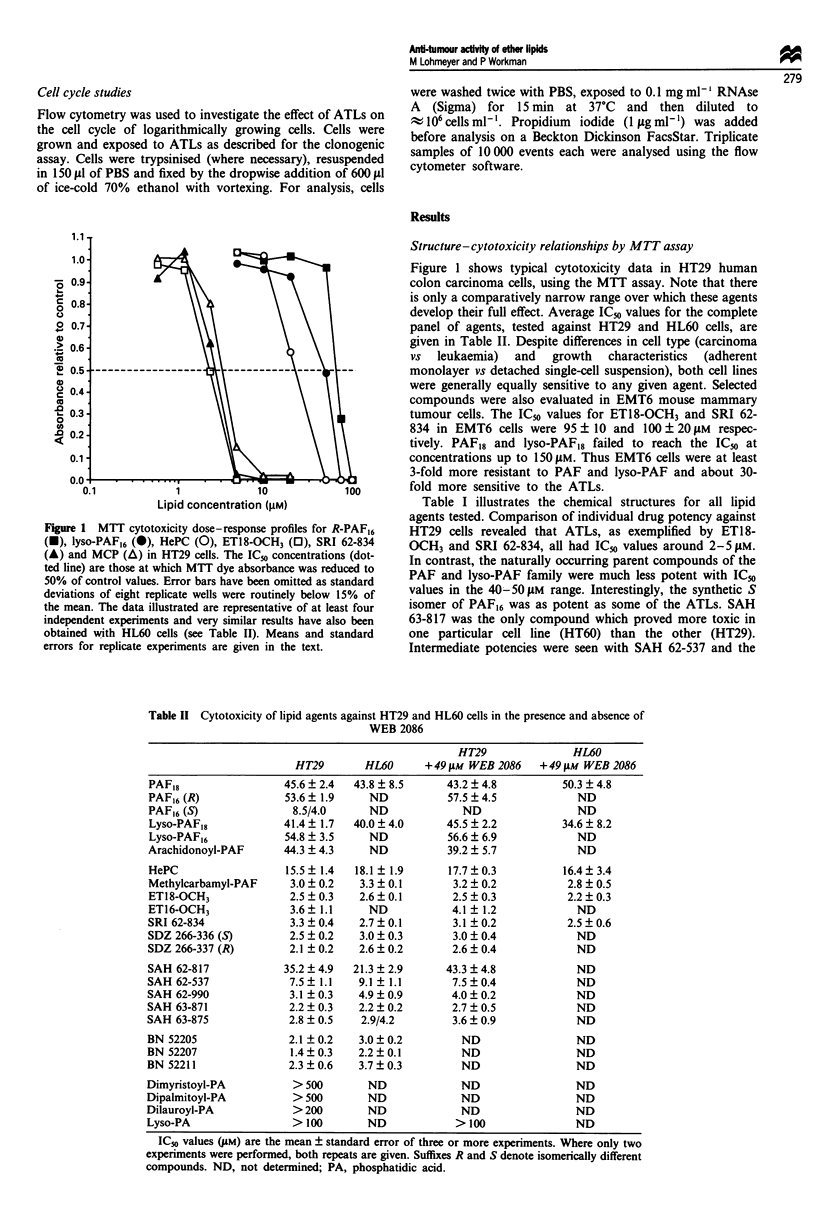
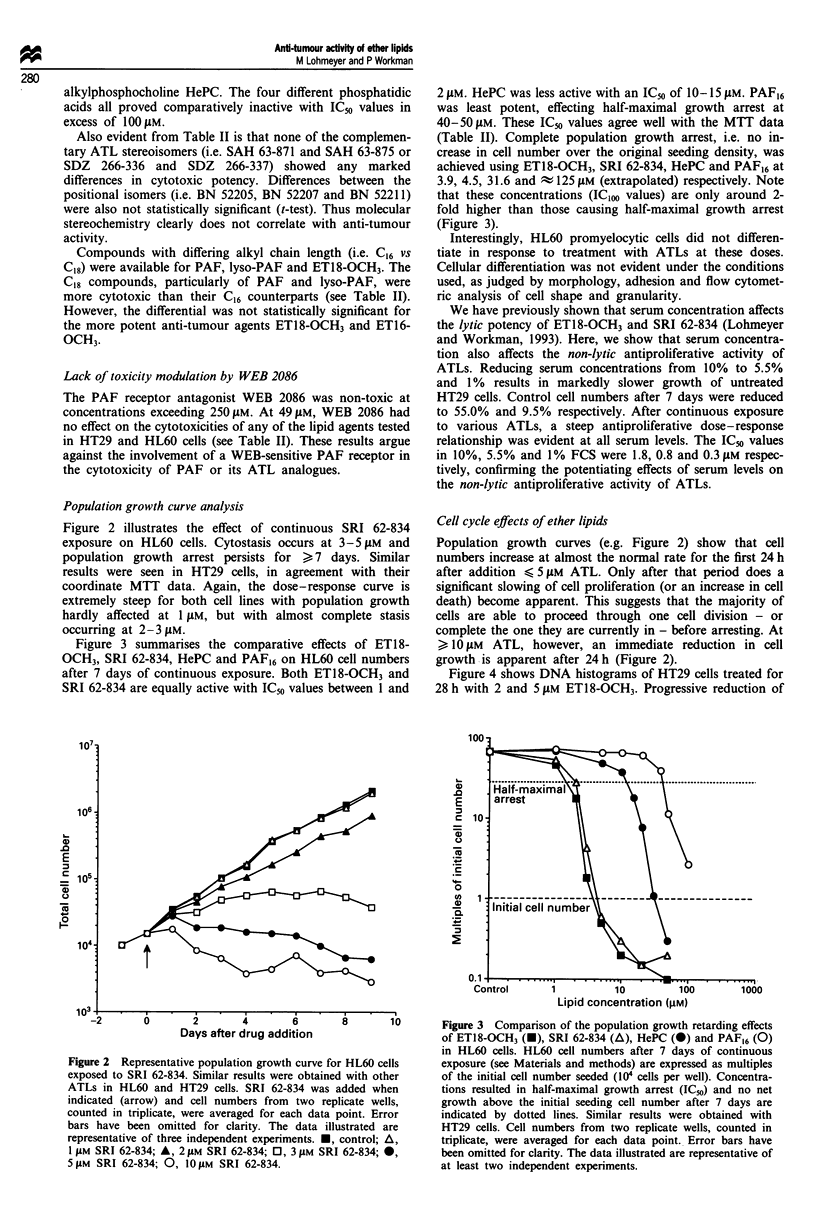
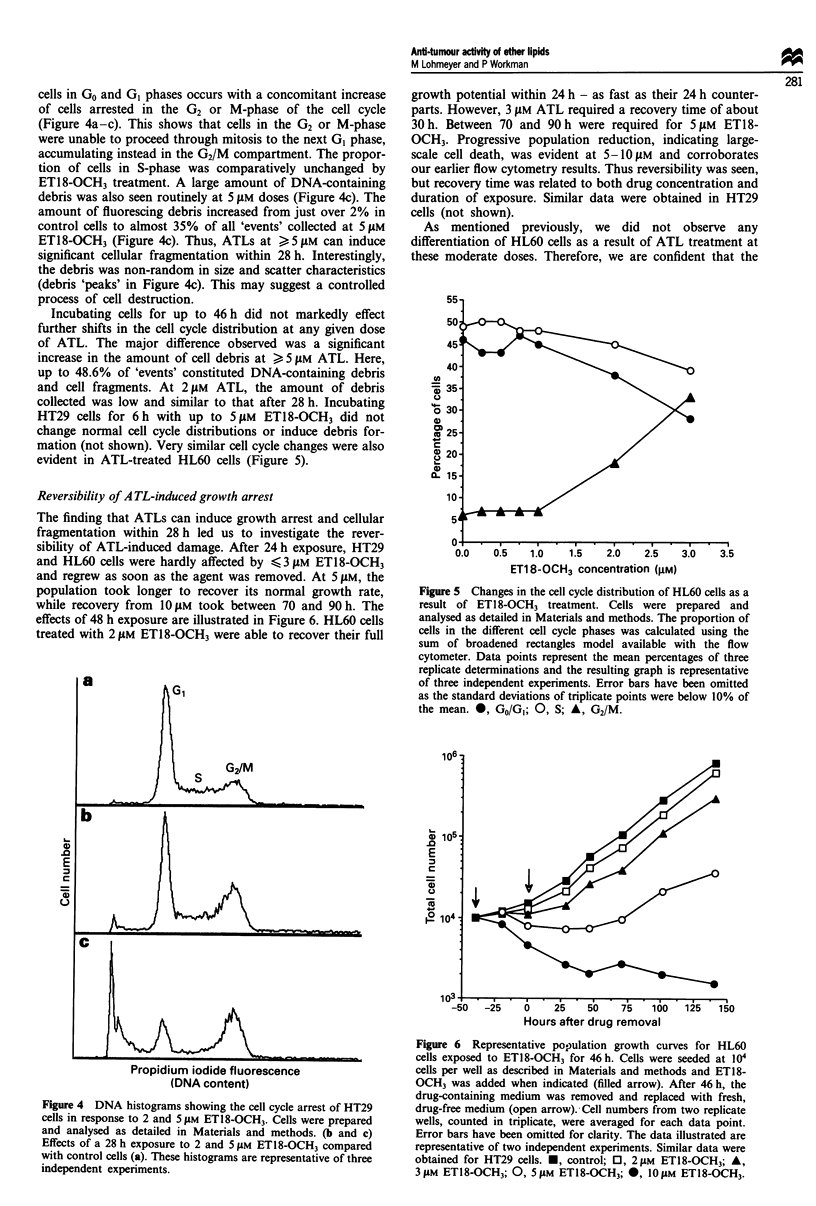
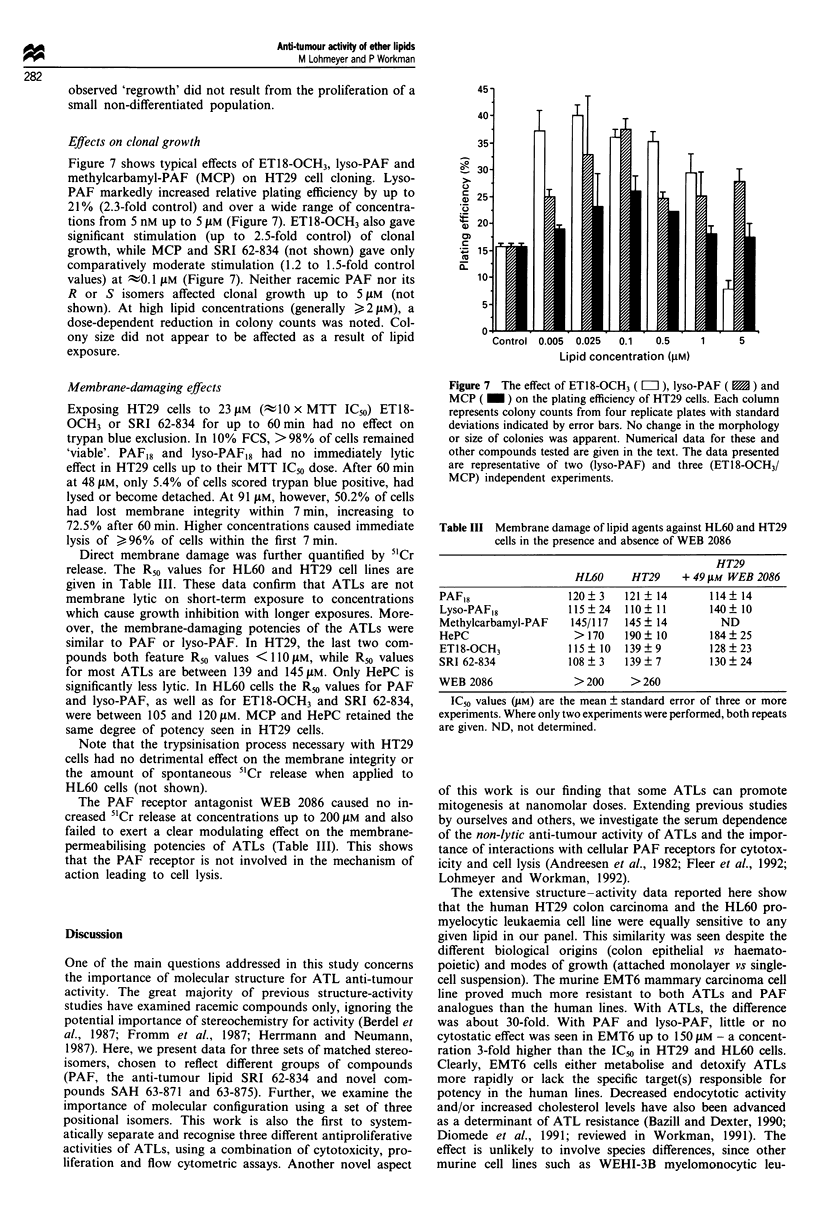
A panel of 25 different lipid agents was evaluated for in vitro activity against HT29 human colon carcinoma and HL60 promyelocytic leukaemia cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The structure-activity relationships seen with this series, including those for four sets of positional or stereoisomers, indicate that specific receptor proteins are unlikely as targets for anti-tumour lipid (ATL) action. Additional data confirm the lack of involvement of the platelet-activating factor receptor in particular and suggest that metabolic stability is a most important determinant of ATL activity. More detailed studies, with 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (ET18-OCH3) and (+/-)-2-(Hydroxy[tetrahydro-2-(octadecyloxy)methylfuran-2- yl]methoxyphosphinyloxy)-N,N,N,-trimethylethaniminium hydroxide (SRI 62-834), suggest three different modes of activity, depending on drug concentration and exposure time. Low doses of up to 5 microM in standard serum-containing medium cause population growth arrest after prolonged exposure. Growth arrest was associated with a leaky G2/M block as determined by flow cytometry. These effects are reversible. Intermediate concentrations (5-40 microM) were cytotoxic, causing a net reduction in cell numbers after 2-3 days. At even higher concentrations, all lipids caused rapid, direct membrane lysis. When the clonogenic assay was used to assess the effects of ATLs, most agents reduced colony formation at concentrations above 5 microM. However, some compounds proved stimulatory at nanomolar concentrations, suggesting that they might possess mitogenic properties. These results, particularly those concerning the concentration and time dependence, may be relevant to current clinical trials with ether lipids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Modolell M., Oepke G. H., Common H., Löhr G. W., Munder P. G. Studies on various parameters influencing leukemic cell destruction by alkyl-lysophospholipids. Anticancer Res. 1982 Jan-Apr;2(1-2):95–100. [PubMed] [Google Scholar]
- Arnold B., Reuther R., Weltzien H. U. Distribution and metabolism of synthetic alkyl analogs of lysophosphatidylcholine in mice. Biochim Biophys Acta. 1978 Jul 25;530(1):47–55. doi: 10.1016/0005-2760(78)90125-x. [DOI] [PubMed] [Google Scholar]
- Bazill G. W., Dexter T. M. Role of endocytosis in the action of ether lipids on WEHI-3B, HL60, and FDCP-mix A4 cells. Cancer Res. 1990 Dec 1;50(23):7505–7512. [PubMed] [Google Scholar]
- Berdel W. E., Bausert W. R., Weltzien H. U., Modolell M. L., Widmann K. H., Munder P. G. The influence of alkyl-lysophospholipids and lysophospholipid-activated macrophages on the development of metastasis of 3-Lewis lung carcinoma. Eur J Cancer. 1980 Sep;16(9):1199–1204. doi: 10.1016/0014-2964(80)90179-6. [DOI] [PubMed] [Google Scholar]
- Berdel W. E. Ether lipids and derivatives as investigational anticancer drugs. A brief review. Onkologie. 1990 Aug;13(4):245–250. doi: 10.1159/000216771. [DOI] [PubMed] [Google Scholar]
- Berdel W. E., Korth R., Reichert A., Houlihan W. J., Bicker U., Nomura H., Vogler W. R., Benveniste J., Rastetter J. Lack of correlation between cytotoxicity of agonists and antagonists of platelet activating factor [paf-acether] in neoplastic cells and modulation of [3H]-paf-acether binding to platelets from humans in vitro. Anticancer Res. 1987 Nov-Dec;7(6):1181–1187. [PubMed] [Google Scholar]
- Berdel W. E. Membrane-interactive lipids as experimental anticancer drugs. Br J Cancer. 1991 Aug;64(2):208–211. doi: 10.1038/bjc.1991.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren M. I., Gallegos A., Dressler L. A., Modest E. J., Powis G. Inhibition of the signalling enzyme phosphatidylinositol-3-kinase by antitumor ether lipid analogues. Cancer Res. 1993 Sep 15;53(18):4297–4302. [PubMed] [Google Scholar]
- Berkovic D., Fleer E. A., Eibl H., Unger C. Effects of hexadecylphosphocholine on cellular function. Prog Exp Tumor Res. 1992;34:59–68. doi: 10.1159/000420832. [DOI] [PubMed] [Google Scholar]
- Bishop F. E., Dive C., Freeman S., Gescher A. Is metabolism an important arbiter of anticancer activity of ether lipids? Metabolism of SRI 62-834 and hexadecylphosphocholine by [31P]-NMR spectroscopy and comparison of their cytotoxicities with those of their metabolites. Cancer Chemother Pharmacol. 1992;31(2):85–92. doi: 10.1007/BF00685092. [DOI] [PubMed] [Google Scholar]
- Breiser A., Kim D. J., Fleer E. A., Damenz W., Drube A., Berger M., Nagel G. A., Eibl H., Unger C. Distribution and metabolism of hexadecylphosphocholine in mice. Lipids. 1987 Nov;22(11):925–926. doi: 10.1007/BF02535556. [DOI] [PubMed] [Google Scholar]
- Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther. 1987 Jun;241(3):974–981. [PubMed] [Google Scholar]
- Danhauser-Riedl S., Felix S. B., Houlihan W. J., Zafferani M., Steinhauser G., Oberberg D., Kalvelage H., Busch R., Rastetter J., Berdel W. E. Some antagonists of platelet activating factor are cytotoxic for human malignant cell lines. Cancer Res. 1991 Jan 1;51(1):43–48. [PubMed] [Google Scholar]
- Diomede L., Bianchi R., Modest E. J., Piovani B., Bubba F., Salmona M. Modulation of ATPase activity by cholesterol and synthetic ether lipids in leukemic cells. Biochem Pharmacol. 1992 Feb 18;43(4):803–807. doi: 10.1016/0006-2952(92)90246-f. [DOI] [PubMed] [Google Scholar]
- Diomede L., Bizzi A., Magistrelli A., Modest E. J., Salmona M., Noseda A. Role of cell cholesterol in modulating antineoplastic ether lipid uptake, membrane effects and cytotoxicity. Int J Cancer. 1990 Aug 15;46(2):341–346. doi: 10.1002/ijc.2910460234. [DOI] [PubMed] [Google Scholar]
- Diomede L., Colotta F., Piovani B., Re F., Modest E. J., Salmona M. Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine. A possible basis for its selective action. Int J Cancer. 1993 Jan 2;53(1):124–130. doi: 10.1002/ijc.2910530123. [DOI] [PubMed] [Google Scholar]
- Diomede L., Piovani B., Modest E. J., Noseda A., Salmona M. Increased ether lipid cytotoxicity by reducing membrane cholesterol content. Int J Cancer. 1991 Sep 30;49(3):409–413. doi: 10.1002/ijc.2910490317. [DOI] [PubMed] [Google Scholar]
- Diomede L., Piovani B., Re F., Principe P., Colotta F., Modest E. J., Salmona M. The induction of apoptosis is a common feature of the cytotoxic action of ether-linked glycerophospholipids in human leukemic cells. Int J Cancer. 1994 Jun 1;57(5):645–649. doi: 10.1002/ijc.2910570506. [DOI] [PubMed] [Google Scholar]
- Dive C., Watson J. V., Workman P. Multiparametric flow cytometry of the modulation of tumor cell membrane permeability by developmental antitumor ether lipid SRI 62-834 in EMT6 mouse mammary tumor and HL-60 human promyelocytic leukemia cells. Cancer Res. 1991 Feb 1;51(3):799–806. [PubMed] [Google Scholar]
- Dummer R., Röger J., Vogt T., Becker J., Hefner H., Sindermann H., Burg G. Topical application of hexadecylphosphocholine in patients with cutaneous lymphomas. Prog Exp Tumor Res. 1992;34:160–169. doi: 10.1159/000420841. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., Leon B. T., Menger F. M., Kuo J. F. Evidence for the regulation of the activity of protein kinase C through changes in membrane properties. Biosci Rep. 1991 Feb;11(1):59–64. doi: 10.1007/BF01118606. [DOI] [PubMed] [Google Scholar]
- Fleer E. A., Berkovic D., Unger C., Eibl H. Cellular uptake and metabolic fate of hexadecylphosphocholine. Prog Exp Tumor Res. 1992;34:33–46. doi: 10.1159/000420830. [DOI] [PubMed] [Google Scholar]
- Fleer E. A., Kim D. J., Nagel G. A., Eibl H., Unger C. Cytotoxic activity of lysophosphatidylcholine analogues on human lymphoma Raji cells. Onkologie. 1990 Aug;13(4):295–300. doi: 10.1159/000216779. [DOI] [PubMed] [Google Scholar]
- Fromm M., Berdel W. E., Schick H. D., Fink U., Pahlke W., Bicker U., Reichert A., Rastetter J. Antineoplastic activity of the thioether lysophospholipid derivative BM 41.440 in vitro. Lipids. 1987 Nov;22(11):916–918. doi: 10.1007/BF02535554. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Grunicke H. H. The cell membrane as a target for cancer chemotherapy. Eur J Cancer. 1991;27(3):281–284. doi: 10.1016/0277-5379(91)90516-g. [DOI] [PubMed] [Google Scholar]
- Herrmann D. B., Neumann H. A. Cytotoxic activity of the thioether phospholipid analogue BM 41.440 in primary human tumor cultures. Lipids. 1987 Nov;22(11):955–957. doi: 10.1007/BF02535563. [DOI] [PubMed] [Google Scholar]
- Hilgard P., Kampherm E., Nolan L., Pohl J., Reissmann T. Investigation into the immunological effects of miltefosine, a new anticancer agent under development. J Cancer Res Clin Oncol. 1991;117(5):403–408. doi: 10.1007/BF01612758. [DOI] [PubMed] [Google Scholar]
- Hoffman D. R., Hajdu J., Snyder F. Cytotoxicity of platelet activating factor and related alkyl-phospholipid analogs in human leukemia cells, polymorphonuclear neutrophils, and skin fibroblasts. Blood. 1984 Mar;63(3):545–552. [PubMed] [Google Scholar]
- Hoffman D. R., Hoffman L. H., Snyder F. Cytotoxicity and metabolism of alkyl phospholipid analogues in neoplastic cells. Cancer Res. 1986 Nov;46(11):5803–5809. [PubMed] [Google Scholar]
- Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991 Jan 24;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Honma Y., Kasukabe T., Hozumi M., Akimoto H., Nomura H. Induction of differentiation of human myeloid leukemia HL-60 cells by novel nonphosphorus alkyl ether lipids. Lipids. 1991 Dec;26(12):1445–1449. doi: 10.1007/BF02536583. [DOI] [PubMed] [Google Scholar]
- Honma Y., Kasukabe T., Hozumi M., Tsushima S., Nomura H. Induction of differentiation of cultured human and mouse myeloid leukemia cells by alkyl-lysophospholipids. Cancer Res. 1981 Aug;41(8):3211–3216. [PubMed] [Google Scholar]
- Houlihan W. J., Lee M. L., Munder P. G., Nemecek G. M., Handley D. A., Winslow C. M., Happy J., Jaeggi C. Antitumor activity of SRI 62-834, a cyclic ether analog of ET-18-OCH3. Lipids. 1987 Nov;22(11):884–890. doi: 10.1007/BF02535549. [DOI] [PubMed] [Google Scholar]
- Houlihan W. J., Lohmeyer M., Workman P., Cheon S. H. Phospholipid antitumor agents. Med Res Rev. 1995 May;15(3):157–223. doi: 10.1002/med.2610150302. [DOI] [PubMed] [Google Scholar]
- Ishaq K. S., Capobianco M., Piantadosi C., Noseda A., Daniel L. W., Modest E. J. Synthesis and biological evaluation of ether-linked derivatives of phosphatidylinositol. Pharm Res. 1989 Mar;6(3):216–224. doi: 10.1023/a:1015961416370. [DOI] [PubMed] [Google Scholar]
- Kantar A., Giorgi P. L., Curatola G., Fiorini R. Effect of PAF on erythrocyte membrane heterogeneity: a fluorescence study. Agents Actions. 1991 Mar;32(3-4):347–350. doi: 10.1007/BF01980897. [DOI] [PubMed] [Google Scholar]
- Kasukabe T., Honma Y., Hozumi M., Nomura H. Inhibition of proliferation and induction of differentiation of human and mouse myeloid leukemia cells by new ethyleneglycol-type nonphosphorus alkyl ether lipids. Jpn J Cancer Res. 1990 Aug;81(8):807–812. doi: 10.1111/j.1349-7006.1990.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosano H., Takatani O. Inhibition by an alkyl-lysophospholipid of the uptake of epidermal growth factor in human breast cancer cell lines in relation to epidermal growth factor internalization. Cancer Res. 1989 Jun 1;49(11):2868–2870. [PubMed] [Google Scholar]
- Kucera L. S., Iyer N., Leake E., Raben A., Modest E. J., Daniel L. W., Piantadosi C. Novel membrane-interactive ether lipid analogs that inhibit infectious HIV-1 production and induce defective virus formation. AIDS Res Hum Retroviruses. 1990 Apr;6(4):491–501. doi: 10.1089/aid.1990.6.491. [DOI] [PubMed] [Google Scholar]
- Lazenby C. M., Thompson M. G., Hickman J. A. Elevation of leukemic cell intracellular calcium by the ether lipid SRI 62-834. Cancer Res. 1990 Jun 1;50(11):3327–3330. [PubMed] [Google Scholar]
- Lohmeyer M., Workman P. Lack of enantio-selectivity in the in vitro antitumour cytotoxicity and membrane-damaging activity of ether lipid SRI 62-834: further evidence for a non-receptor-mediated mechanism of action. Biochem Pharmacol. 1992 Aug 18;44(4):819–823. doi: 10.1016/0006-2952(92)90421-e. [DOI] [PubMed] [Google Scholar]
- Lohmeyer M., Workman P. The role of intracellular free calcium mobilization in the mechanism of action of antitumour ether lipids SRI 62-834 and ET18-OMe. Biochem Pharmacol. 1993 Jan 7;45(1):77–86. doi: 10.1016/0006-2952(93)90379-b. [DOI] [PubMed] [Google Scholar]
- Mangold H. K., Weber N. Biosynthesis and biotransformation of ether lipids. Lipids. 1987 Nov;22(11):789–799. doi: 10.1007/BF02535533. [DOI] [PubMed] [Google Scholar]
- Marasco C. J., Jr, Piantadosi C., Meyer K. L., Morris-Natschke S., Ishaq K. S., Small G. W., Daniel L. W. Synthesis and biological activity of novel quaternary ammonium derivatives of alkylglycerols as potent inhibitors of protein kinase C. J Med Chem. 1990 Mar;33(3):985–992. doi: 10.1021/jm00165a016. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Hilgard P. Induction of tumor cell differentiation by alkylphosphocholines: a new approach for in vitro screening. Prog Exp Tumor Res. 1992;34:90–97. doi: 10.1159/000420835. [DOI] [PubMed] [Google Scholar]
- Mende S., Teuscher E., Windeck I., Lichtnow K. H., Nuhn P., Brachwitz H. Zum Einfluss von Alkyllysophospholipiden auf Membranpotential, Teilung und Migration von isolierten Kälberaortenendothelzellen. Pharmazie. 1989 Oct;44(10):713–715. [PubMed] [Google Scholar]
- Meyer K. L., Marasco C. J., Jr, Morris-Natschke S. L., Ishaq K. S., Piantadosi C. In vitro evaluation of phosphocholine and quaternary ammonium containing lipids as novel anti-HIV agents. J Med Chem. 1991 Apr;34(4):1377–1383. doi: 10.1021/jm00108a021. [DOI] [PubMed] [Google Scholar]
- Modolell M., Andreesen R., Pahlke W., Brugger U., Munder P. G. Disturbance of phospholipid metabolism during the selective destruction of tumor cells induced by alkyl-lysophospholipids. Cancer Res. 1979 Nov;39(11):4681–4686. [PubMed] [Google Scholar]
- Morimoto H., Broquet C., Principe P., Mencia-Huerta J. M., Braquet P., Bonavida B. Cytotoxic activity of synthetic aza alkyl lysophospholipids against drug sensitive and drug resistant human tumor cell lines. Anticancer Res. 1991 Nov-Dec;11(6):2223–2229. [PubMed] [Google Scholar]
- Munder P. G., Westphal O. Antitumoral and other biomedical activities of synthetic ether lysophospholipids. Chem Immunol. 1990;49:206–235. [PubMed] [Google Scholar]
- Nakagawa Y., Waku K. The metabolism of glycerophospholipid and its regulation in monocytes and macrophages. Prog Lipid Res. 1989;28(3):205–243. doi: 10.1016/0163-7827(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Noseda A., Berens M. E., White J. G., Modest E. J. In vitro antiproliferative activity of combinations of ether lipid analogues and DNA-interactive agents against human tumor cells. Cancer Res. 1988 Apr 1;48(7):1788–1791. [PubMed] [Google Scholar]
- Noseda A., Godwin P. L., Modest E. J. Effects of antineoplastic ether lipids on model and biological membranes. Biochim Biophys Acta. 1988 Nov 3;945(1):92–100. doi: 10.1016/0005-2736(88)90366-5. [DOI] [PubMed] [Google Scholar]
- Noseda A., White J. G., Godwin P. L., Jerome W. G., Modest E. J. Membrane damage in leukemic cells induced by ether and ester lipids: an electron microscopic study. Exp Mol Pathol. 1989 Feb;50(1):69–83. doi: 10.1016/0014-4800(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Oishi K., Zheng B., White J. F., Vogler W. R., Kuo J. F. Inhibition of Na,K-ATPase and sodium pump by anticancer ether lipids and protein kinase C inhibitors ET-18-OCH3 and BM 41.440. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1000–1006. doi: 10.1016/s0006-291x(88)80973-2. [DOI] [PubMed] [Google Scholar]
- Page C., Abbott A. PAF: new antagonists, new roles in disease and a major role in reproductive biology. Trends Pharmacol Sci. 1989 Jul;10(7):255–257. doi: 10.1016/0165-6147(89)90017-5. [DOI] [PubMed] [Google Scholar]
- Pignol B., Chaumeron S., Coulomb H., Maisonnet T., Vandamme B., Broquet C., Mencia-Huerta J. M., Braquet P. Immunomodulatory activity of two new aza alkyl phospholipid antineoplastic drugs. Anticancer Drugs. 1992 Dec;3(6):599–608. doi: 10.1097/00001813-199212000-00007. [DOI] [PubMed] [Google Scholar]
- Powis G., Seewald M. J., Gratas C., Melder D., Riebow J., Modest E. J. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992 May 15;52(10):2835–2840. [PubMed] [Google Scholar]
- Principe P., Sidoti C., Braquet P. Tumor cell kinetics following antineoplastic ether phospholipid treatment. Cancer Res. 1992 May 1;52(9):2509–2515. [PubMed] [Google Scholar]
- Raynor R. L., Zheng B., Kuo J. F. Membrane interactions of amphiphilic polypeptides mastoparan, melittin, polymyxin B, and cardiotoxin. Differential inhibition of protein kinase C, Ca2+/calmodulin-dependent protein kinase II and synaptosomal membrane Na,K-ATPase, and Na+ pump and differentiation of HL60 cells. J Biol Chem. 1991 Feb 15;266(5):2753–2758. [PubMed] [Google Scholar]
- Sawyer D. B., Andersen O. S. Platelet-activating factor is a general membrane perturbant. Biochim Biophys Acta. 1989 Dec 11;987(1):129–132. doi: 10.1016/0005-2736(89)90464-1. [DOI] [PubMed] [Google Scholar]
- Seewald M. J., Olsen R. A., Sehgal I., Melder D. C., Modest E. J., Powis G. Inhibition of growth factor-dependent inositol phosphate Ca2+ signaling by antitumor ether lipid analogues. Cancer Res. 1990 Aug 1;50(15):4458–4463. [PubMed] [Google Scholar]
- Shoji M., Raynor R. L., Berdel W. E., Vogler W. R., Kuo J. F. Effects of thioether phospholipid BM 41.440 on protein kinase C and phorbol ester-induced differentiation of human leukemia HL60 and KG-1 cells. Cancer Res. 1988 Dec 1;48(23):6669–6673. [PubMed] [Google Scholar]
- Sleight R. G., Abanto M. N. Differences in intracellular transport of a fluorescent phosphatidylcholine analog in established cell lines. J Cell Sci. 1989 Jun;93(Pt 2):363–374. doi: 10.1242/jcs.93.2.363. [DOI] [PubMed] [Google Scholar]
- Sobottka S. B., Berger M. R., Eibl H. Structure-activity relationships of four anti-cancer alkylphosphocholine derivatives in vitro and in vivo. Int J Cancer. 1993 Feb 1;53(3):418–425. doi: 10.1002/ijc.2910530312. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Schneider M., Lenz B., Phillips H., Long C. Immunomodulatory and therapeutic properties of alkyl lysophospholipids in mice. Lipids. 1987 Nov;22(11):871–877. doi: 10.1007/BF02535547. [DOI] [PubMed] [Google Scholar]
- Uberall F., Oberhuber H., Maly K., Zaknun J., Demuth L., Grunicke H. H. Hexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activity. Cancer Res. 1991 Feb 1;51(3):807–812. [PubMed] [Google Scholar]
- Unger C., Sindermann H., Peukert M., Hilgard P., Engel J., Eibl H. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Prog Exp Tumor Res. 1992;34:153–159. doi: 10.1159/000420840. [DOI] [PubMed] [Google Scholar]
- Verweij J., Krzemieniecki K., Kok T., Poveda A., van Pottelsberghe C., van Glabbeke M., Mouridsen H. Phase II study of miltefosine (hexadecylphosphocholine) in advanced soft tissue sarcomas of the adult--an EORTC Soft Tissue and Bone Sarcoma Group Study. Eur J Cancer. 1993;29A(2):208–209. doi: 10.1016/0959-8049(93)90177-h. [DOI] [PubMed] [Google Scholar]
- Vichi P., Tritton T. R. Stimulation of growth in human and murine cells by adriamycin. Cancer Res. 1989 May 15;49(10):2679–2682. [PubMed] [Google Scholar]
- Vogler W. R. Bone marrow purging in acute leukemia with alkyl-lysophospholipids: a new family of anticancer drugs. Leuk Lymphoma. 1994 Mar;13(1-2):53–60. doi: 10.3109/10428199409051652. [DOI] [PubMed] [Google Scholar]
- Vogler W. R., Olson A. C., Hajdu J., Shoji M., Raynor R., Kuo J. F. Structure-function relationships of alkyl-lysophospholipid analogs in selective antitumor activity. Lipids. 1993 Jun;28(6):511–516. doi: 10.1007/BF02536082. [DOI] [PubMed] [Google Scholar]
- Workman P. Antitumor ether lipids: endocytosis as a determinant of cellular sensitivity. Cancer Cells. 1991 Aug;3(8):315–317. [PubMed] [Google Scholar]
- Workman P., Donaldson J., Lohmeyer M. Platelet-activating factor (PAF) antagonist WEB 2086 does not modulate the cytotoxicity of PAF or antitumour alkyl lysophospholipids ET-18-O-methyl and SRI 62-834 in HL-60 promyelocytic leukaemia cells. Biochem Pharmacol. 1991 Jan 15;41(2):319–322. doi: 10.1016/0006-2952(91)90496-r. [DOI] [PubMed] [Google Scholar]


