Abstract
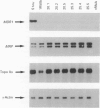
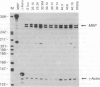
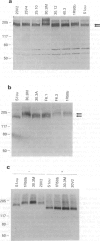
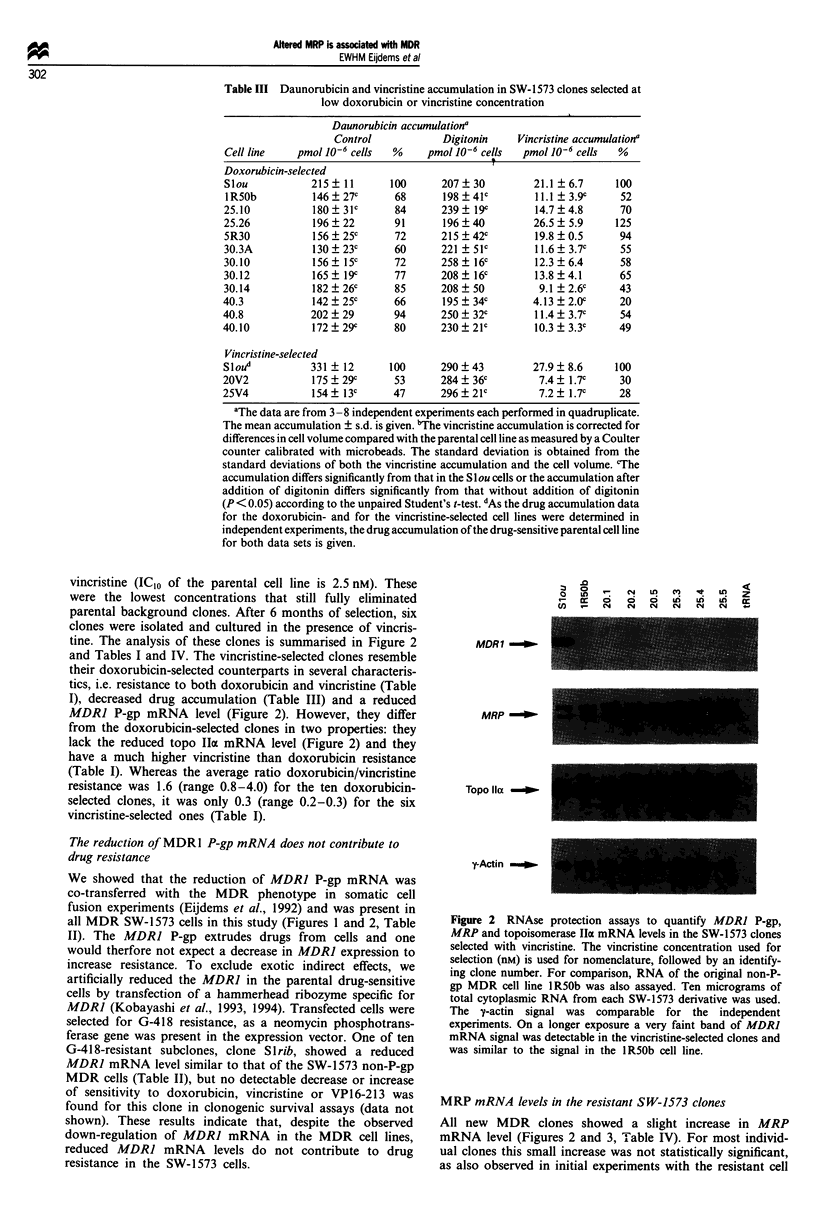
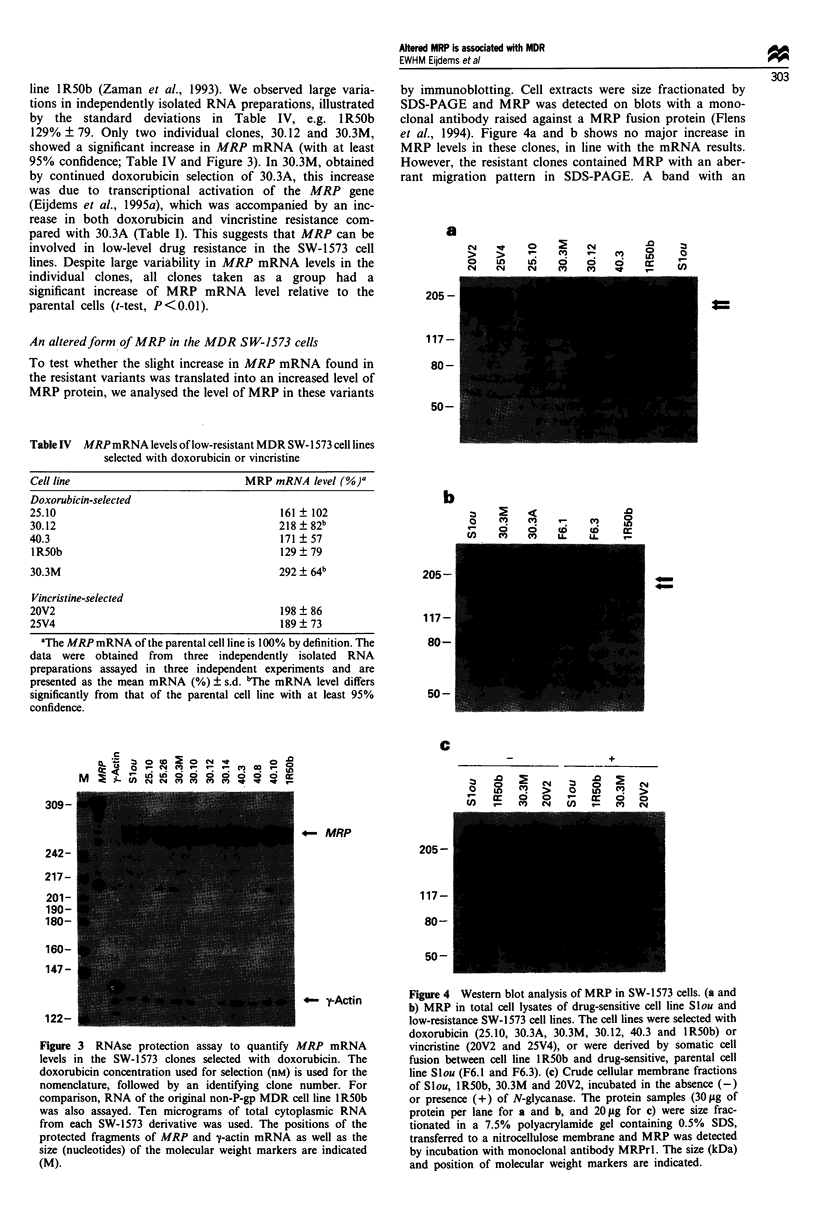
We have analysed the contribution of several parameters, e.g. drug accumulation, MDR1 P-glycoprotein (P-gp), multidrug resistance-associated protein (MRP) and topoisomerase (topo) II, to drug resistance in a large set of drug-resistant variants of the human non-small-cell lung cancer cell line SW-1573 derived by selection with low concentrations of doxorubicin or vincristine. Selection with either drug nearly always resulted in MDR clones. The resistance of these clones could be explained by reduced drug accumulation and was associated with a decrease rather than an increase in the low MDR1 mRNA level. To test whether a decrease in MDR1 mRNA indirectly affected resistance in these cells, we introduced a MDR1-specific hammerhead ribozyme into wild-type SW-1573 cells. Although this led to a substantial reduction in MDR1 mRNA, it did not result in resistance. In all resistant clones we found an altered form of the multidrug resistance-associated protein (MRP), migrating slightly slower during SDS-polyacrylamide gel electrophoresis than MRP in parental cells. This altered MRP was also present in non-P-gp MDR somatic cell hybrids of the SW-1573 cells, demonstrating a clear linkage with the MDR phenotype. Treatment of crude cellular membrane fractions with N-glycanase, endoglycosidase H or neuraminidase showed that the altered migration of MRP on SDS-PAGE is due to a post-translational modification. There was no detectable difference in sialic acid content. In most but not all doxorubicin-selected clones, this MDR phenotype was accompanied by a reduction in topo II alpha mRNA level. No reduction was found in the clones selected with vincristine. We conclude from these results that selection of the SW-1573 cell line for low levels of doxorubicin or vincristine resistance, predominantly results in MDR with reduced drug accumulation associated with the presence of an altered MRP protein. This mechanism can be accompanied by other resistance mechanisms, such as reduced topo II alpha mRNA in case of doxorubicin selection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Barrand M. A., Heppell-Parton A. C., Wright K. A., Rabbitts P. H., Twentyman P. R. A 190-kilodalton protein overexpressed in non-P-glycoprotein-containing multidrug-resistant cells and its relationship to the MRP gene. J Natl Cancer Inst. 1994 Jan 19;86(2):110–117. doi: 10.1093/jnci/86.2.110. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Kuiper C. M., Schuurhuis G. J., Tsuruo T., Pinedo H. M., Lankelma J. Increase of daunorubicin and vincristine accumulation in multidrug resistant human ovarian carcinoma cells by a monoclonal antibody reacting with P-glycoprotein. Biochem Pharmacol. 1988 Jun 15;37(12):2389–2393. doi: 10.1016/0006-2952(88)90365-6. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Chanda E. R., Dicke F. P., Gerlach J. H., Mirski S. E. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991 Jul 1;51(13):3345–3352. [PubMed] [Google Scholar]
- Cole S. P. The 1991 Merck Frosst Award. Multidrug resistance in small cell lung cancer. Can J Physiol Pharmacol. 1992 Mar;70(3):313–329. doi: 10.1139/y92-040. [DOI] [PubMed] [Google Scholar]
- Eijdems E. W., Borst P., Jongsma A. P., de Jong S., de Vries E. G., van Groenigen M., Versantvoort C. H., Nieuwint A. W., Baas F. Genetic transfer of non-P-glycoprotein-mediated multidrug resistance (MDR) in somatic cell fusion: dissection of a compound MDR phenotype. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3498–3502. doi: 10.1073/pnas.89.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijdems E. W., De Haas M., Coco-Martin J. M., Ottenheim C. P., Zaman G. J., Dauwerse H. G., Breuning M. H., Twentyman P. R., Borst P., Baas F. Mechanisms of MRP over-expression in four human lung-cancer cell lines and analysis of the MRP amplicon. Int J Cancer. 1995 Mar 3;60(5):676–684. doi: 10.1002/ijc.2910600518. [DOI] [PubMed] [Google Scholar]
- Eijdems E. W., de Haas M., Timmerman A. J., Van der Schans G. P., Kamst E., de Nooij J., Astaldi Ricotti G. C., Borst P., Baas F. Reduced topoisomerase II activity in multidrug-resistant human non-small cell lung cancer cell lines. Br J Cancer. 1995 Jan;71(1):40–47. doi: 10.1038/bjc.1995.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Gerlach J. H., Bell D. R., Karakousis C., Slocum H. K., Kartner N., Rustum Y. M., Ling V., Baker R. M. P-glycoprotein in human sarcoma: evidence for multidrug resistance. J Clin Oncol. 1987 Sep;5(9):1452–1460. doi: 10.1200/JCO.1987.5.9.1452. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grant C. E., Valdimarsson G., Hipfner D. R., Almquist K. C., Cole S. P., Deeley R. G. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994 Jan 15;54(2):357–361. [PubMed] [Google Scholar]
- Keizer H. G., Schuurhuis G. J., Broxterman H. J., Lankelma J., Schoonen W. G., van Rijn J., Pinedo H. M., Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989 Jun 1;49(11):2988–2993. [PubMed] [Google Scholar]
- Kobayashi H., Dorai T., Holland J. F., Ohnuma T. Cleavage of human MDR1 mRNA by a hammerhead ribozyme. FEBS Lett. 1993 Mar 15;319(1-2):71–74. doi: 10.1016/0014-5793(93)80039-w. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Dorai T., Holland J. F., Ohnuma T. Reversal of drug sensitivity in multidrug-resistant tumor cells by an MDR1 (PGY1) ribozyme. Cancer Res. 1994 Mar 1;54(5):1271–1275. [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Moscow J. A., Schneider E., Cowan K. H. Multidrug resistance. Cancer Chemother Biol Response Modif. 1993;14:98–117. [PubMed] [Google Scholar]
- Schinkel A. H., Borst P. Multidrug resistance mediated by P-glycoproteins. Semin Cancer Biol. 1991 Aug;2(4):213–226. [PubMed] [Google Scholar]
- Schinkel A. H., Kemp S., Dollé M., Rudenko G., Wagenaar E. N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem. 1993 Apr 5;268(10):7474–7481. [PubMed] [Google Scholar]
- Schneider E., Horton J. K., Yang C. H., Nakagawa M., Cowan K. H. Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 1994 Jan 1;54(1):152–158. [PubMed] [Google Scholar]
- Slovak M. L., Ho J. P., Bhardwaj G., Kurz E. U., Deeley R. G., Cole S. P. Localization of a novel multidrug resistance-associated gene in the HT1080/DR4 and H69AR human tumor cell lines. Cancer Res. 1993 Jul 15;53(14):3221–3225. [PubMed] [Google Scholar]
- Tsai-Pflugfelder M., Liu L. F., Liu A. A., Tewey K. M., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Wang J. C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Feller N., Dekker H., Kuiper C. M., Lankelma J. Probing daunorubicin accumulation defects in non-P-glycoprotein expressing multidrug-resistant cell lines using digitonin. Int J Cancer. 1992 Apr 1;50(6):906–911. doi: 10.1002/ijc.2910500615. [DOI] [PubMed] [Google Scholar]
- Zaman G. J., Flens M. J., van Leusden M. R., de Haas M., Mülder H. S., Lankelma J., Pinedo H. M., Scheper R. J., Baas F., Broxterman H. J. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G. J., Versantvoort C. H., Smit J. J., Eijdems E. W., de Haas M., Smith A. J., Broxterman H. J., Mulder N. H., de Vries E. G., Baas F. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993 Apr 15;53(8):1747–1750. [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]