Abstract
Inhibitors of eucaryotes (cycloheximide and amphotericin B) and procaryotes (penicillin and chloramphenicol) were used to estimate bacterivory and bacterial production in a eutrophic lake. Bacterial production appeared to be slightly greater than protozoan grazing in the aerobic waters of Lake Oglethorpe. Use of penicillin and cycloheximide yielded inconsistent results in anaerobic water and in aerobic water when bacterial production was low. Production measured by inhibiting eucaryotes with cycloheximide did not always agree with [3H]thymidine estimates or differential filtration methods. Laboratory experiments showed that several common freshwater protozoans continued to swim and ingest bacterium-size latex beads in the presence of the eucaryote inhibitor. Penicillin also affected grazing rates of some ciliates. We recommend that caution and a corroborating method be used when estimating ecologically important parameters with specific inhibitors.
Full text
PDF



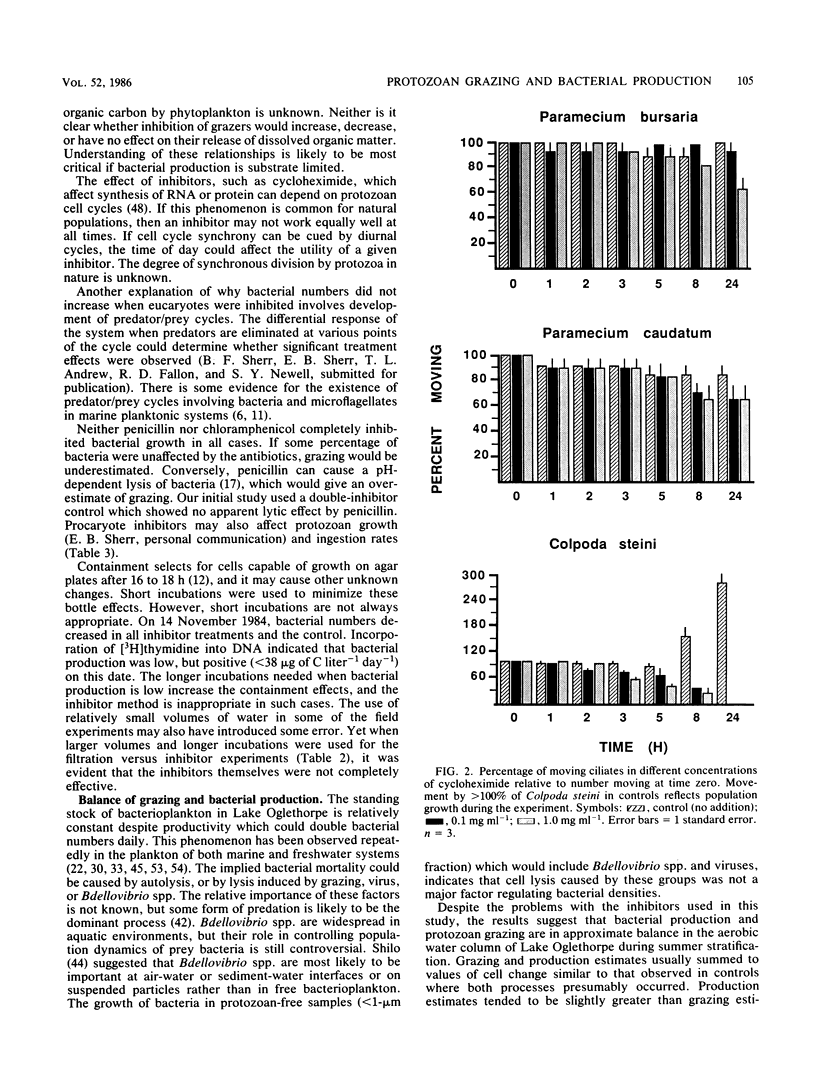
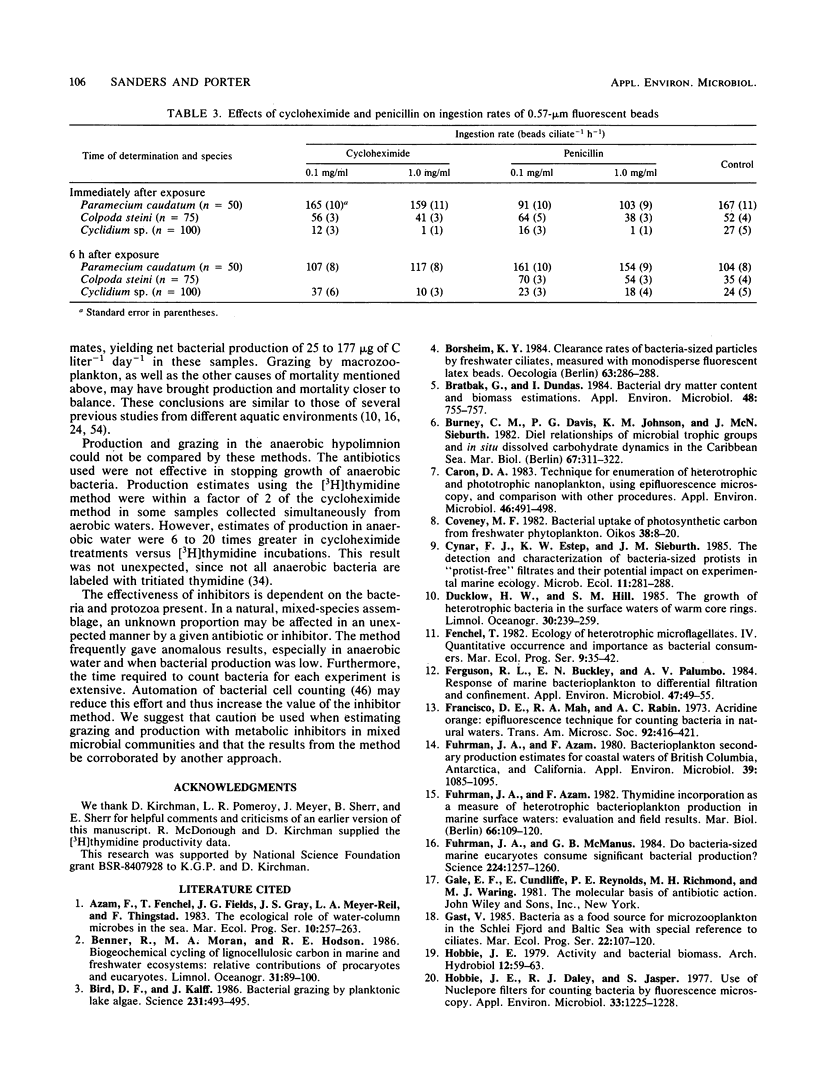

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird D. F., Kalff J. Bacterial grazing by planktonic lake algae. Science. 1986 Jan 31;231(4737):493–495. doi: 10.1126/science.231.4737.493. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984 Oct;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McManus G. B. Do bacteria-sized marine eukaryotes consume significant bacterial production? Science. 1984 Jun 15;224(4654):1257–1260. doi: 10.1126/science.224.4654.1257. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Holm H. W. Rates of transformation of methyl parathion and diethyl phthalate by aufwuchs microorganisms. Appl Environ Microbiol. 1981 Oct;42(4):698–703. doi: 10.1128/aem.42.4.698-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J., McMeekin T. A. Relative effects of bacterial and protozoan predators on survival of Escherichia coli in estuarine water samples. Appl Environ Microbiol. 1980 Nov;40(5):907–911. doi: 10.1128/aem.40.5.907-911.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez C., Alexander M. Evidence Suggesting Protozoan Predation on Rhizobium Associated with Germinating Seeds and in the Rhizosphere of Beans (Phaseolus vulgaris L.). Appl Environ Microbiol. 1980 Sep;40(3):492–499. doi: 10.1128/aem.40.3.492-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais P., Billen G., Rego J. V. Rate of bacterial mortality in aquatic environments. Appl Environ Microbiol. 1985 Jun;49(6):1448–1454. doi: 10.1128/aem.49.6.1448-1454.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Berman T. Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl Environ Microbiol. 1983 Apr;45(4):1196–1201. doi: 10.1128/aem.45.4.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieracki M. E., Johnson P. W., Sieburth J. M. Detection, enumeration, and sizing of planktonic bacteria by image-analyzed epifluorescence microscopy. Appl Environ Microbiol. 1985 Apr;49(4):799–810. doi: 10.1128/aem.49.4.799-810.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhama M., Hanson E. D. The role of protein synthesis in prefission morphogenesis of Paramecium aurelia. J Exp Zool. 1971 Aug;177(4):463–478. doi: 10.1002/jez.1401770408. [DOI] [PubMed] [Google Scholar]



