Abstract
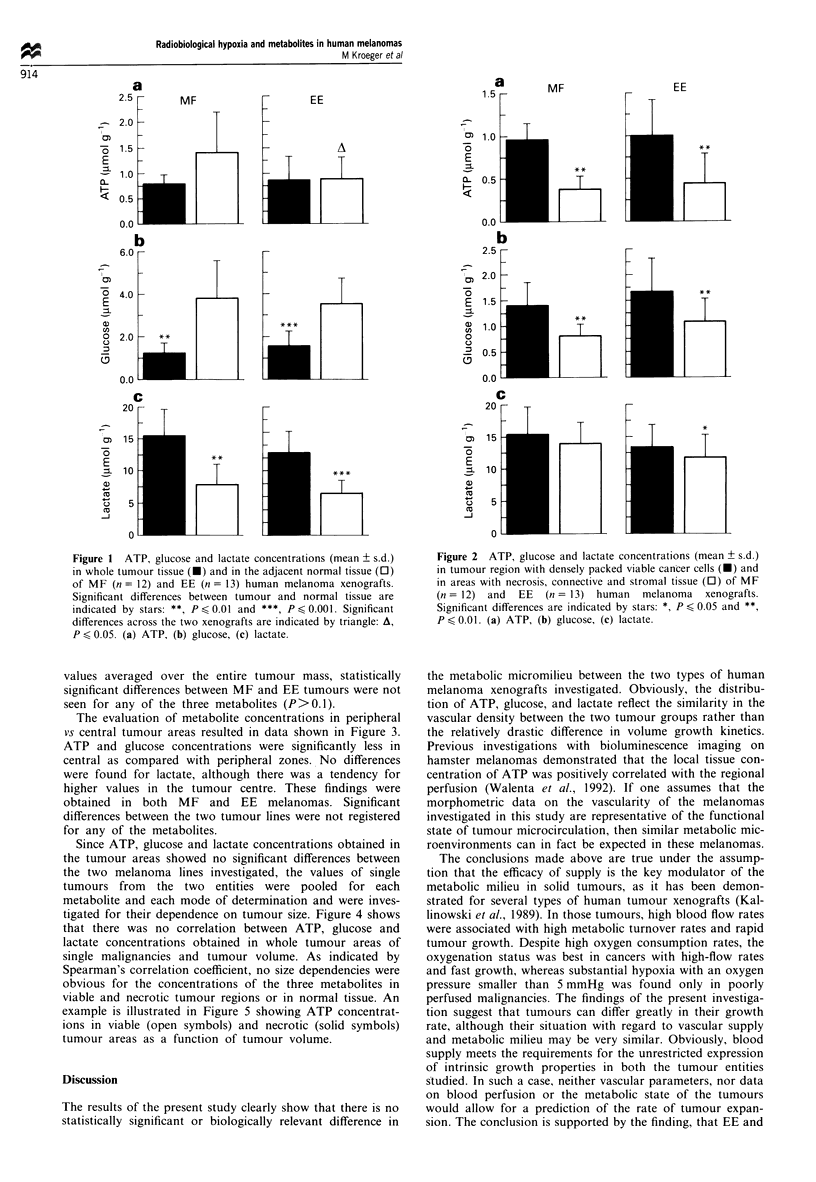
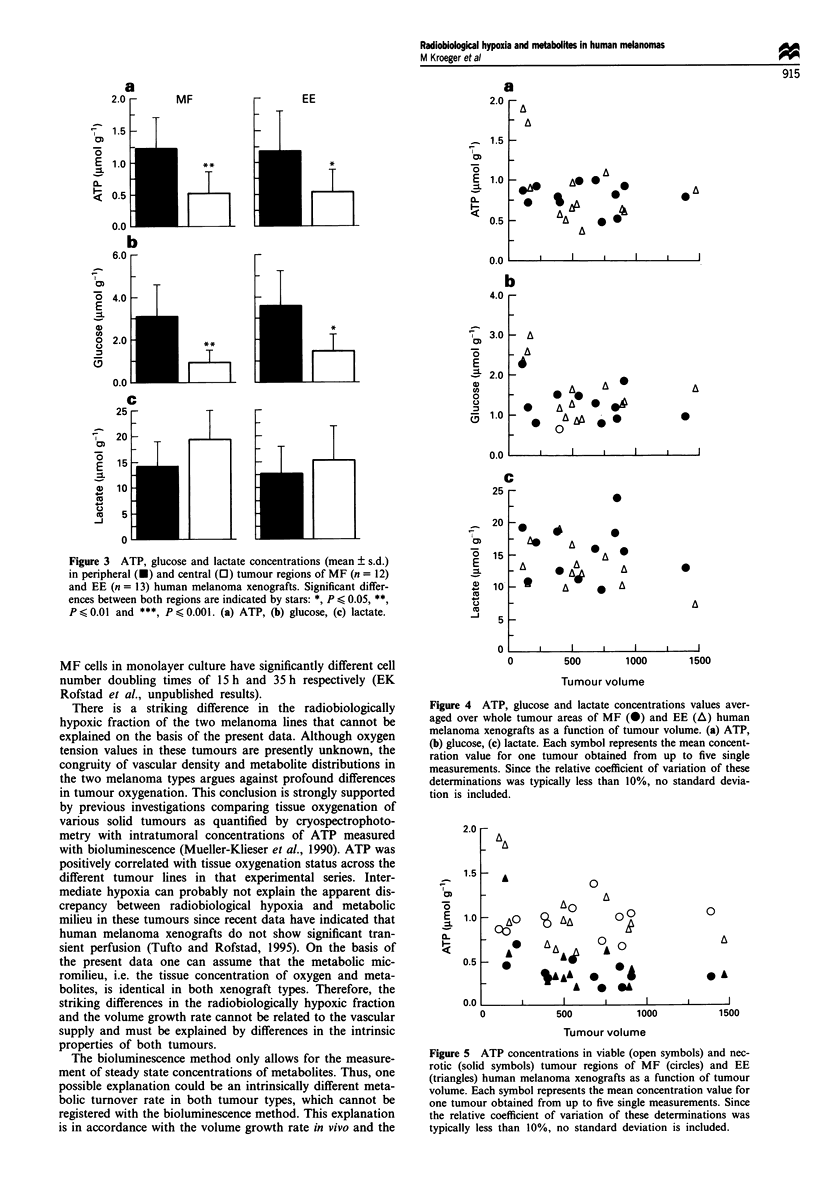
Investigations were carried out on two lines of human melanomas (MF; n = 12 and EE; n = 13) xenografted in nude mice. The tumours were characterised by a similar vascular supply but showed a pronounced difference in the rate of volume growth and in the radiobiologically hypoxic fraction. The distribution of ATP, glucose and lactate in the tumours was investigated using quantitative bioluminescence and single photon imaging. Concentrations of the metabolites were obtained as global values for the entire tumour mass, in regions with densely packed, structurally intact tumour cells ('viable zones'), in areas with necrosis, stromal cells and fibrous material ('necrotic zones') and in adjacent normal tissue. In all melanomas investigated glucose concentrations were significantly lower and lactate concentrations were significantly higher than in normal tissue. In contrast, no significant differences for ATP were detected. ATP and glucose concentrations were significantly less in necrotic than in viable tumour zones, whereas lactate concentrations were nearly equal in these tumour parts. Corresponding results were obtained in central versus peripheral tumour zones. There was no dependency of global or regional metabolite concentrations on tumour size within the volume range 110-1470 mm3. Based on this lack of dependency, metabolic concentrations were averaged over the whole tumour size range. Metabolite concentrations were not significantly different either globally or regionally between the two tumour entities investigated, a finding which held true for all three metabolites registered. Thus, metabolite distributions apparently mirror the similarity in vascularity of MF and EE melanomas rather than reflecting intrinsic properties with regard to tumour growth rates or susceptibility to radiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Höckel M., Schlenger K., Knoop C., Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991 Nov 15;51(22):6098–6102. [PubMed] [Google Scholar]
- Kallinowski F., Schlenger K. H., Runkel S., Kloes M., Stohrer M., Okunieff P., Vaupel P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989 Jul 15;49(14):3759–3764. [PubMed] [Google Scholar]
- Kuhnle G. E., Dellian M., Walenta S., Mueller-Klieser W., Goetz A. E. Simultaneous high-resolution measurement of adenosine triphosphate levels and blood flow in the hamster amelanotic melanoma A-Mel-3. J Natl Cancer Inst. 1992 Nov 4;84(21):1642–1647. doi: 10.1093/jnci/84.21.1642. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W., Schaefer C., Walenta S., Rofstad E. K., Fenton B. M., Sutherland R. M. Assessment of tumor energy and oxygenation status by bioluminescence, nuclear magnetic resonance spectroscopy, and cryospectrophotometry. Cancer Res. 1990 Mar 15;50(6):1681–1685. [PubMed] [Google Scholar]
- Mueller-Klieser W., Walenta S. Geographical mapping of metabolites in biological tissue with quantitative bioluminescence and single photon imaging. Histochem J. 1993 Jun;25(6):407–420. doi: 10.1007/BF00157805. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W., Walenta S., Paschen W., Kallinowski F., Vaupel P. Metabolic imaging in microregions of tumors and normal tissues with bioluminescence and photon counting. J Natl Cancer Inst. 1988 Aug 3;80(11):842–848. doi: 10.1093/jnci/80.11.842. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., Brustad T. Arrhenius analysis of the heat response in vivo and in vitro of human melanoma xenografts. Int J Hyperthermia. 1986 Oct-Dec;2(4):359–368. doi: 10.3109/02656738609004966. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., Brustad T. Radiation response in vitro of cells from five human malignant melanoma xenografts. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Dec;40(6):677–680. doi: 10.1080/09553008114551671. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Growth and vascular structure of human melanoma xenografts. Cell Tissue Kinet. 1984 Jan;17(1):91–101. doi: 10.1111/j.1365-2184.1984.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Hypoxia and reoxygenation in human melanoma xenografts. Int J Radiat Oncol Biol Phys. 1989 Jul;17(1):81–89. doi: 10.1016/0360-3016(89)90374-x. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Local tumor control following single dose irradiation of human melanoma xenografts: relationship to cellular radiosensitivity and influence of an immune response by the athymic mouse. Cancer Res. 1989 Jun 15;49(12):3163–3167. [PubMed] [Google Scholar]
- Schaefer C., Mayer W. K., Krüger W., Vaupel P. Microregional distributions of glucose, lactate, ATP and tissue pH in experimental tumours upon local hyperthermia and/or hyperglycaemia. J Cancer Res Clin Oncol. 1993;119(10):599–608. doi: 10.1007/BF01372723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solesvik O. V., Rofstad E. K., Brustad T. Vascular changes in a human malignant melanoma xenograft following single-dose irradiation. Radiat Res. 1984 Apr;98(1):115–128. [PubMed] [Google Scholar]
- Solesvik O. V., Rofstad E. K., Brustad T. Vascular structure of five human malignant melanomas grown in athymic nude mice. Br J Cancer. 1982 Oct;46(4):557–567. doi: 10.1038/bjc.1982.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufto I., Rofstad E. K. Transient perfusion in human melanoma xenografts. Br J Cancer. 1995 Apr;71(4):789–793. doi: 10.1038/bjc.1995.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]
- Walenta S., Dellian M., Goetz A. E., Kuhnle G. E., Mueller-Klieser W. Pixel-to-pixel correlation between images of absolute ATP concentrations and blood flow in tumours. Br J Cancer. 1992 Dec;66(6):1099–1102. doi: 10.1038/bjc.1992.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenta S., Dötsch J., Mueller-Klieser W. ATP concentrations in multicellular tumor spheroids assessed by single photon imaging and quantitative bioluminescence. Eur J Cell Biol. 1990 Aug;52(2):389–393. [PubMed] [Google Scholar]


