Abstract
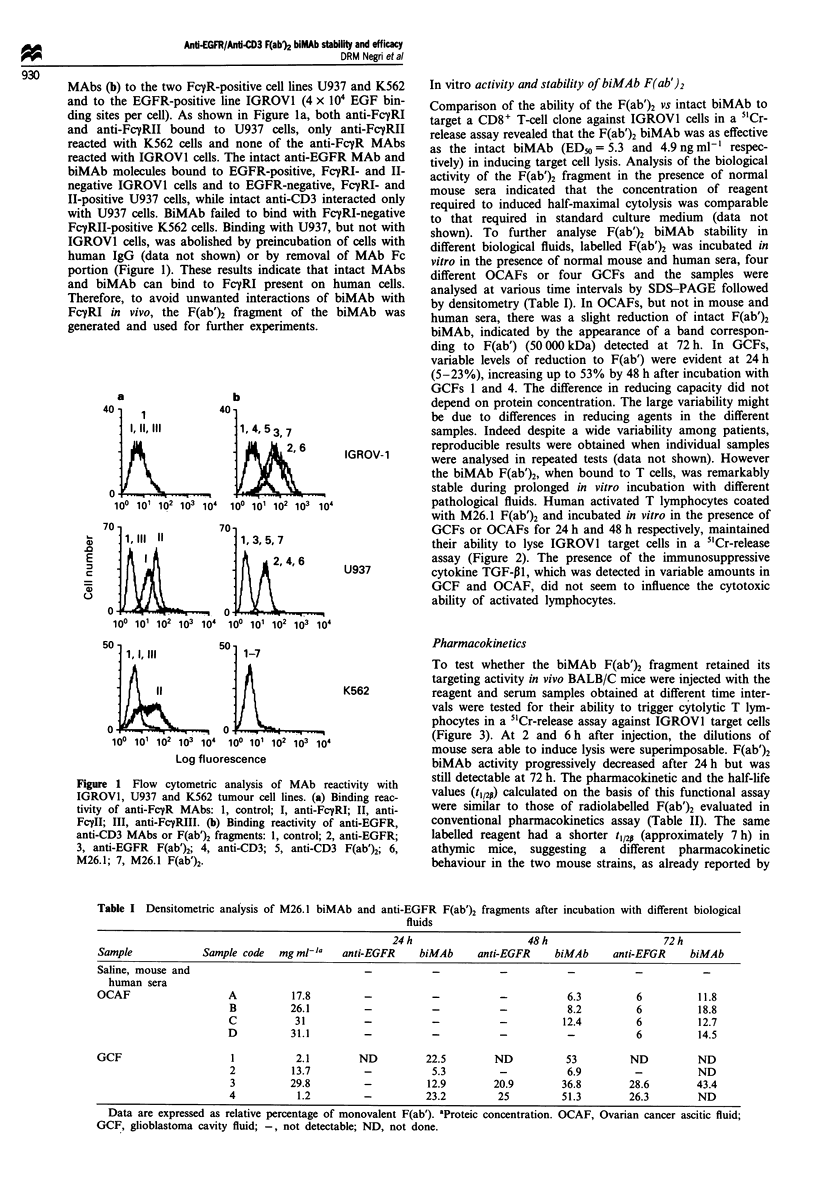
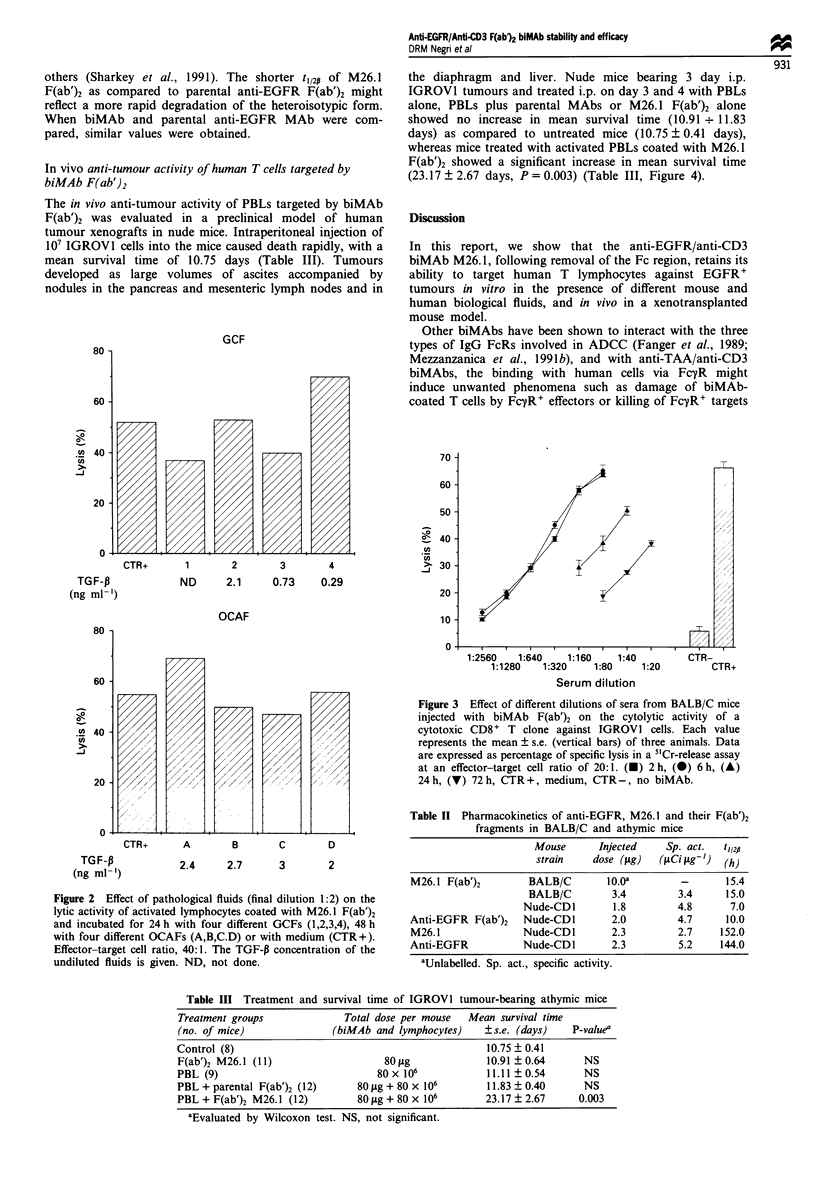
The in vitro and in vivo stability and anti-tumour efficacy of the anti-EGFR/anti-CD3 bispecific monoclonal antibody (biMAb), M26.1, were analysed. The interaction of the intact biMAb with Fc receptor I (Fc gamma RI) present on human leucocytes was not observed when the antibody was used as an F(ab')2 fragment. A CD8+ T-cell clone coated with M26.1 F(ab')2 was as effective as the intact biMAb in inducing IGROV1 target cell lysis when tested in a 51Cr-release assay. Variable levels of reduction of F(ab')2 to monovalent F(ab') were observed upon incubation with human ovarian cancer ascitic fluid (OCAF) or with human glioblastoma cavity fluid (GCF), but not with mouse or human sera. Activated lymphocytes coated with F(ab')2 and incubated in vitro with GCF or OCAF for 24 and 48 h respectively maintained their targeting. Thus, the F(ab')2, when present as a soluble molecule, but not when bound to T cells, might lose some functional activity as a consequence of partial reduction to F(ab'). In normal mice, M26.1 F(ab')2 retained full cytotoxic activity in the circulation, and clearance values were similar to those obtained with parental and other MAb F(ab')2. Treatment of IGROV1 tumour-bearing mice with activated human lymphocytes coated with the M26.1 F(ab')2 significantly prolonged survival of the animals compared with tumour-bearing untreated and control mice treated with lymphocytes or F(ab')2 alone. Together, these results suggest the clinical usefulness of bispecific M26.1 F(ab')2 as a targeting agent for local treatment of tumours such as glioma and ovarian cancers that express variable levels of epidermal growth factor receptor (EGFR).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks-Schlegel S. P., Quintero J. Human esophageal carcinoma cells have fewer, but higher affinity epidermal growth factor receptors. J Biol Chem. 1986 Apr 5;261(10):4359–4362. [PubMed] [Google Scholar]
- Baselga J., Norton L., Masui H., Pandiella A., Coplan K., Miller W. H., Jr, Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993 Aug 18;85(16):1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- Bender H., Takahashi H., Adachi K., Belser P., Liang S. H., Prewett M., Schrappe M., Sutter A., Rodeck U., Herlyn D. Immunotherapy of human glioma xenografts with unlabeled, 131I-, or 125I-labeled monoclonal antibody 425 to epidermal growth factor receptor. Cancer Res. 1992 Jan 1;52(1):121–126. [PubMed] [Google Scholar]
- Beun G. D., van de Velde C. J., Fleuren G. J. T-cell based cancer immunotherapy: direct or redirected tumor-cell recognition? Immunol Today. 1994 Jan;15(1):11–15. doi: 10.1016/0167-5699(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Bolhuis R. L., Lamers C. H., Goey S. H., Eggermont A. M., Trimbos J. B., Stoter G., Lanzavecchia A., di Re E., Miotti S., Raspagliesi F. Adoptive immunotherapy of ovarian carcinoma with bs-MAb-targeted lymphocytes: a multicenter study. Int J Cancer Suppl. 1992;7:78–81. [PubMed] [Google Scholar]
- Brady L. W., Miyamoto C., Woo D. V., Rackover M., Emrich J., Bender H., Dadparvar S., Steplewski Z., Koprowski H., Black P. Malignant astrocytomas treated with iodine-125 labeled monoclonal antibody 425 against epidermal growth factor receptor: a phase II trial. Int J Radiat Oncol Biol Phys. 1992;22(1):225–230. doi: 10.1016/0360-3016(92)91009-c. [DOI] [PubMed] [Google Scholar]
- Chaffanet M., Chauvin C., Lainé M., Berger F., Chédin M., Rost N., Nissou M. F., Benabid A. L. EGF receptor amplification and expression in human brain tumours. Eur J Cancer. 1992;28(1):11–17. doi: 10.1016/0959-8049(92)90374-b. [DOI] [PubMed] [Google Scholar]
- Divgi C. R., Welt S., Kris M., Real F. X., Yeh S. D., Gralla R., Merchant B., Schweighart S., Unger M., Larson S. M. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991 Jan 16;83(2):97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- Fan Z., Baselga J., Masui H., Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993 Oct 1;53(19):4637–4642. [PubMed] [Google Scholar]
- Fanger M. W., Morganelli P. M., Guyre P. M. Bispecific antibodies. Crit Rev Immunol. 1992;12(3-4):101–124. [PubMed] [Google Scholar]
- Fanger M. W., Shen L., Graziano R. F., Guyre P. M. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989 Mar;10(3):92–99. doi: 10.1016/0167-5699(89)90234-X. [DOI] [PubMed] [Google Scholar]
- Ferrini S., Cambiaggi A., Sforzini S., Marciano S., Canevari S., Mezzanzanica D., Colnaghi M. I., Grossi C. E., Moretta L. Targeting of T lymphocytes against EGF-receptor+ tumor cells by bispecific monoclonal antibodies: requirement of CD3 molecule cross-linking for T-cell activation. Int J Cancer. 1993 Dec 2;55(6):931–937. doi: 10.1002/ijc.2910550610. [DOI] [PubMed] [Google Scholar]
- Ferrini S., Prigione I., Mammoliti S., Colnaghi M. I., Menard S., Moretta A., Moretta L. Retargeting of T-cell-receptor gamma/delta+ lymphocytes against tumor cells by bispecific monoclonal antibodies. Induction of cytolytic activity and lymphokine production. Int J Cancer Suppl. 1989;4:53–55. doi: 10.1002/ijc.2910440714. [DOI] [PubMed] [Google Scholar]
- Fox S. B., Smith K., Hollyer J., Greenall M., Hastrich D., Harris A. L. The epidermal growth factor receptor as a prognostic marker: results of 370 patients and review of 3009 patients. Breast Cancer Res Treat. 1994 Jan;29(1):41–49. doi: 10.1007/BF00666180. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Kawamoto T., Cochet C., Le A., Sato J. D., Masui H., McLeod C., Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Jun 25;259(12):7755–7760. [PubMed] [Google Scholar]
- Gullick W. J. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull. 1991 Jan;47(1):87–98. doi: 10.1093/oxfordjournals.bmb.a072464. [DOI] [PubMed] [Google Scholar]
- Hirte H., Clark D. A. Generation of lymphokine-activated killer cells in human ovarian carcinoma ascitic fluid: identification of transforming growth factor-beta as a suppressive factor. Cancer Immunol Immunother. 1991;32(5):296–302. doi: 10.1007/BF01789047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalofonos H. P., Pawlikowska T. R., Hemingway A., Courtenay-Luck N., Dhokia B., Snook D., Sivolapenko G. B., Hooker G. R., McKenzie C. G., Lavender P. J. Antibody guided diagnosis and therapy of brain gliomas using radiolabeled monoclonal antibodies against epidermal growth factor receptor and placental alkaline phosphatase. J Nucl Med. 1989 Oct;30(10):1636–1645. [PubMed] [Google Scholar]
- Libermann T. A., Nusbaum H. R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M. D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani M., Camagna M., Tarditi L., Seccamani E. A new enzymatic method to obtain high-yield F(ab)2 suitable for clinical use from mouse IgGl. Mol Immunol. 1991 Jan-Feb;28(1-2):69–77. doi: 10.1016/0161-5890(91)90088-2. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J. The epidermal growth factor receptor as a target for therapy with antireceptor monoclonal antibodies. Semin Cancer Biol. 1990 Oct;1(5):339–344. [PubMed] [Google Scholar]
- Mezzanzanica D., Canevari S., Colnaghi M. I. Retargeting of human lymphocytes against human ovarian carcinoma cells by bispecific antibodies: from laboratory to clinic. Int J Clin Lab Res. 1991;21(2):159–164. doi: 10.1007/BF02591636. [DOI] [PubMed] [Google Scholar]
- Mezzanzanica D., Garrido M. A., Neblock D. S., Daddona P. E., Andrew S. M., Zurawski V. R., Jr, Segal D. M., Wunderlich J. R. Human T-lymphocytes targeted against an established human ovarian carcinoma with a bispecific F(ab')2 antibody prolong host survival in a murine xenograft model. Cancer Res. 1991 Oct 15;51(20):5716–5721. [PubMed] [Google Scholar]
- Nitta T., Sato K., Yagita H., Okumura K., Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet. 1990 Feb 17;335(8686):368–371. doi: 10.1016/0140-6736(90)90205-j. [DOI] [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Pupa S. M., Canevari S., Fontanelli R., Ménard S., Mezzanzanica D., Lanzavecchia A., Colnaghi M. I. Activation of mononuclear cells to be used for hybrid monoclonal antibody-induced lysis of human ovarian carcinoma cells. Int J Cancer. 1988 Sep 15;42(3):455–459. doi: 10.1002/ijc.2910420324. [DOI] [PubMed] [Google Scholar]
- Qian J. H., Titus J. A., Andrew S. M., Mezzanzanica D., Garrido M. A., Wunderlich J. R., Segal D. M. Human peripheral blood lymphocytes targeted with bispecific antibodies release cytokines that are essential for inhibiting tumor growth. J Immunol. 1991 May 1;146(9):3250–3256. [PubMed] [Google Scholar]
- Renner C., Jung W., Sahin U., Denfeld R., Pohl C., Trümper L., Hartmann F., Diehl V., van Lier R., Pfreundschuh M. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science. 1994 May 6;264(5160):833–835. doi: 10.1126/science.8171337. [DOI] [PubMed] [Google Scholar]
- Rodeck U., Herlyn M., Herlyn D., Molthoff C., Atkinson B., Varello M., Steplewski Z., Koprowski H. Tumor growth modulation by a monoclonal antibody to the epidermal growth factor receptor: immunologically mediated and effector cell-independent effects. Cancer Res. 1987 Jul 15;47(14):3692–3696. [PubMed] [Google Scholar]
- Rodriguez G. C., Berchuck A., Whitaker R. S., Schlossman D., Clarke-Pearson D. L., Bast R. C., Jr Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. II. Relationship between receptor expression and response to epidermal growth factor. Am J Obstet Gynecol. 1991 Mar;164(3):745–750. doi: 10.1016/0002-9378(91)90508-o. [DOI] [PubMed] [Google Scholar]
- Ruffini P. A., Rivoltini L., Silvani A., Boiardi A., Parmiani G. Factors, including transforming growth factor beta, released in the glioblastoma residual cavity, impair activity of adherent lymphokine-activated killer cells. Cancer Immunol Immunother. 1993 Jun;36(6):409–416. doi: 10.1007/BF01742258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Garrido M. A., Perez P., Titus J. A., Winkler D. A., Ring D. B., Kaubisch A., Wunderlich J. R. Targeted cytotoxic cells as a novel form of cancer immunotherapy. Mol Immunol. 1988 Nov;25(11):1099–1103. doi: 10.1016/0161-5890(88)90144-7. [DOI] [PubMed] [Google Scholar]
- Sharkey R. M., Natale A., Goldenberg D. M., Mattes M. J. Rapid blood clearance of immunoglobulin G2a and immunoglobulin G2b in nude mice. Cancer Res. 1991 Jun 15;51(12):3102–3107. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Ruppert J. M., Bigner S. H., Grzeschik C. H., Humphrey P. A., Bigner D. S., Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk J., Zegveld S. T., Fleuren G. J., Warnaar S. O. Localization of monoclonal antibody G250 and bispecific monoclonal antibody CD3/G250 in human renal-cell carcinoma xenografts: relative effects of size and affinity. Int J Cancer. 1991 Jul 9;48(5):738–743. doi: 10.1002/ijc.2910480518. [DOI] [PubMed] [Google Scholar]


