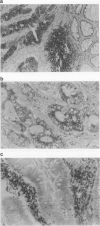
Abstract
The bcl-2 gene encodes for a mitochondrial membrane proto-oncoprotein, the expression of which is known to suppress programmed cell death (apoptosis). In the present study the prognostic value of bcl-2 proto-oncoprotein was immunohistochemically investigated in a series of 104 colorectal carcinomas. The bcl-2 staining patterns were semiquantitatively assessed and correlated with the pTNM stage, Dukes' classification, lymphocytic infiltration (Jass classification) and tumour grade as well as parameters not associated with prognosis (gender, age, tumour site, histological tumour type). Statistical analysis was carried out using the Kaplan-Meier method, the log-rank test, hazard ratios and their confidence intervals. Fifty-five out of 104 cases completely lacked immunohistochemical bcl-2 expression. Fewer than 5% of bcl-2-positive cells were found in 25, 5-50% in 17 and more than 50% in five cases. The extent of bcl-2 expression by tumour cells decreased significantly with respect to increasing tumour size (P < 0.05), decreasing lymphocytic infiltration (P < 0.05) and chance of poor clinical outcome (P < 0.05), but not with worsening of Dukes stages. In multivariate analysis (Cox regression model) bcl-2 expression remained as an independent prognostic parameter with Dukes' classification as stratification factor (P < 0.001). Although the biological functions of bcl-2 protein are not yet well understood, our results provide further evidence that bcl-2 oncoprotein appears to be associated with favourable clinical outcome. Thus immunohistochemical bcl-2 phenotyping of colorectal carcinoma may contribute in future to the clinical management of these patients.
Full text
PDF
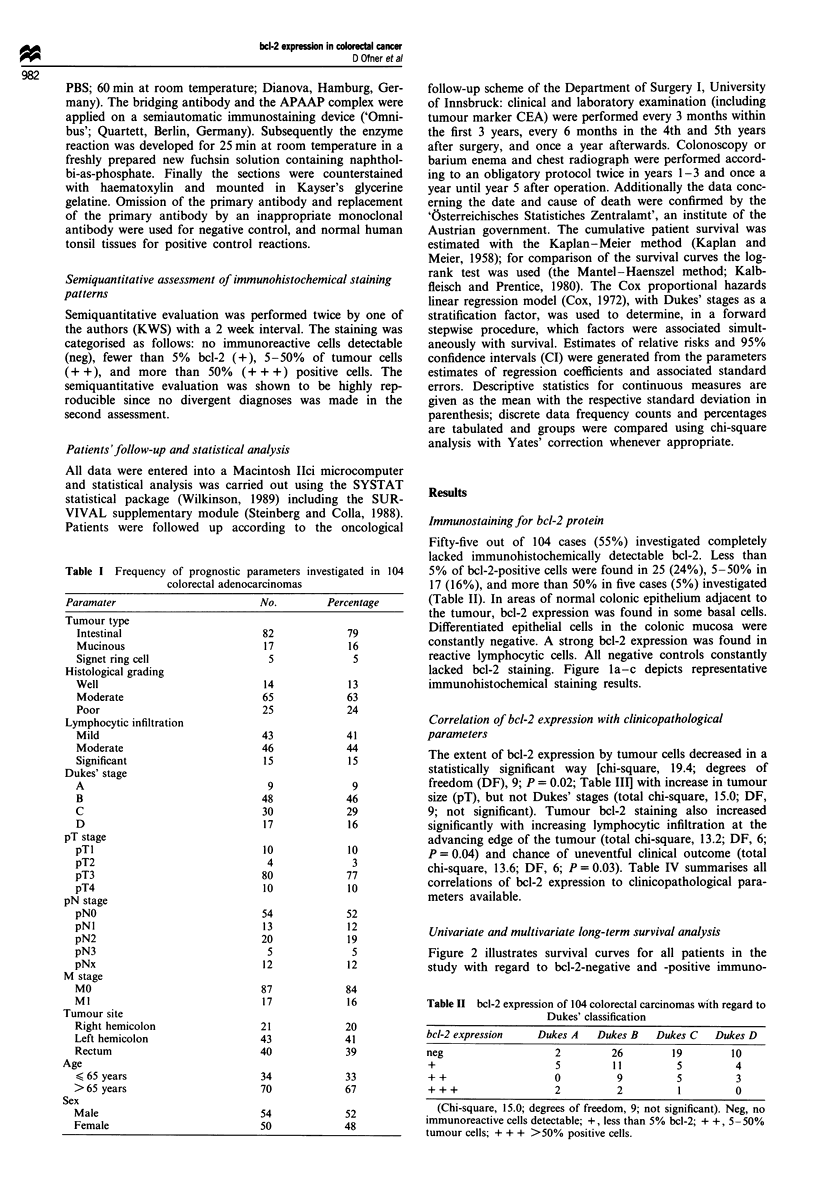
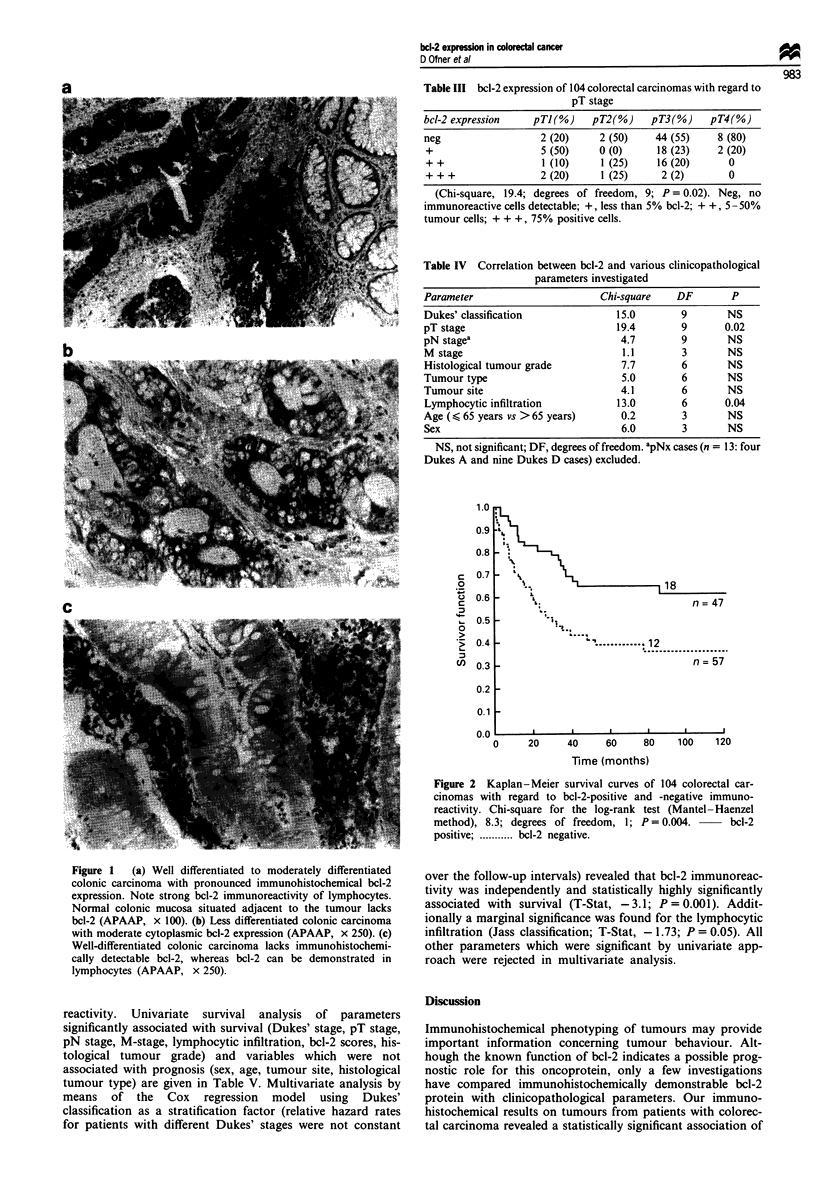


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castle V. P., Heidelberger K. P., Bromberg J., Ou X., Dole M., Nuñez G. Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol. 1993 Dec;143(6):1543–1550. [PMC free article] [PubMed] [Google Scholar]
- Corbally N., Grogan L., Keane M. M., Devaney D. M., Dervan P. A., Carney D. N. Bcl-2 rearrangement in Hodgkin's disease and reactive lymph nodes. Am J Clin Pathol. 1994 Jun;101(6):756–760. doi: 10.1093/ajcp/101.6.756. [DOI] [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doglioni C., Dei Tos A. P., Laurino L., Chiarelli C., Barbareschi M., Viale G. The prevalence of BCL-2 immunoreactivity in breast carcinomas and its clinicopathological correlates, with particular reference to oestrogen receptor status. Virchows Arch. 1994;424(1):47–51. doi: 10.1007/BF00197392. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Atkin W. S., Cuzick J., Bussey H. J., Morson B. C., Northover J. M., Todd I. P. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986 May;10(5):437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Leek R. D., Kaklamanis L., Pezzella F., Gatter K. C., Harris A. L. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994 Jan;69(1):135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P., Robertson D., Amos T. A., Dyer M. J., Mason D. Y., Greaves M. F. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992 Dec;40(12):1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Papadopoulos N., Markowitz S., Willson J. K., Kinzler K. W., Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 1994 Jul 15;54(14):3714–3717. [PubMed] [Google Scholar]
- Pilotti S., Collini P., Del Bo R., Cattoretti G., Pierotti M. A., Rilke F. A novel panel of antibodies that segregates immunocytochemically poorly differentiated carcinoma from undifferentiated carcinoma of the thyroid gland. Am J Surg Pathol. 1994 Oct;18(10):1054–1064. doi: 10.1097/00000478-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Pilotti S., Collini P., Rilke F., Cattoretti G., Del Bo R., Pierotti M. A. Bcl-2 protein expression in carcinomas originating from the follicular epithelium of the thyroid gland. J Pathol. 1994 Apr;172(4):337–342. doi: 10.1002/path.1711720408. [DOI] [PubMed] [Google Scholar]
- Richter C. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett. 1993 Jun 28;325(1-2):104–107. doi: 10.1016/0014-5793(93)81423-w. [DOI] [PubMed] [Google Scholar]
- Silvestrini R., Veneroni S., Daidone M. G., Benini E., Boracchi P., Mezzetti M., Di Fronzo G., Rilke F., Veronesi U. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994 Apr 6;86(7):499–504. doi: 10.1093/jnci/86.7.499. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Zhao M., Zhang N. X., Economou M., Blaha I., Laissue J. A., Zimmermann A. Immunohistochemical detection of bcl-2 protein in liver lesions: bcl-2 protein is expressed in hepatocellular carcinomas but not in liver cell dysplasia. Histopathology. 1994 Sep;25(3):237–245. doi: 10.1111/j.1365-2559.1994.tb01323.x. [DOI] [PubMed] [Google Scholar]