Abstract
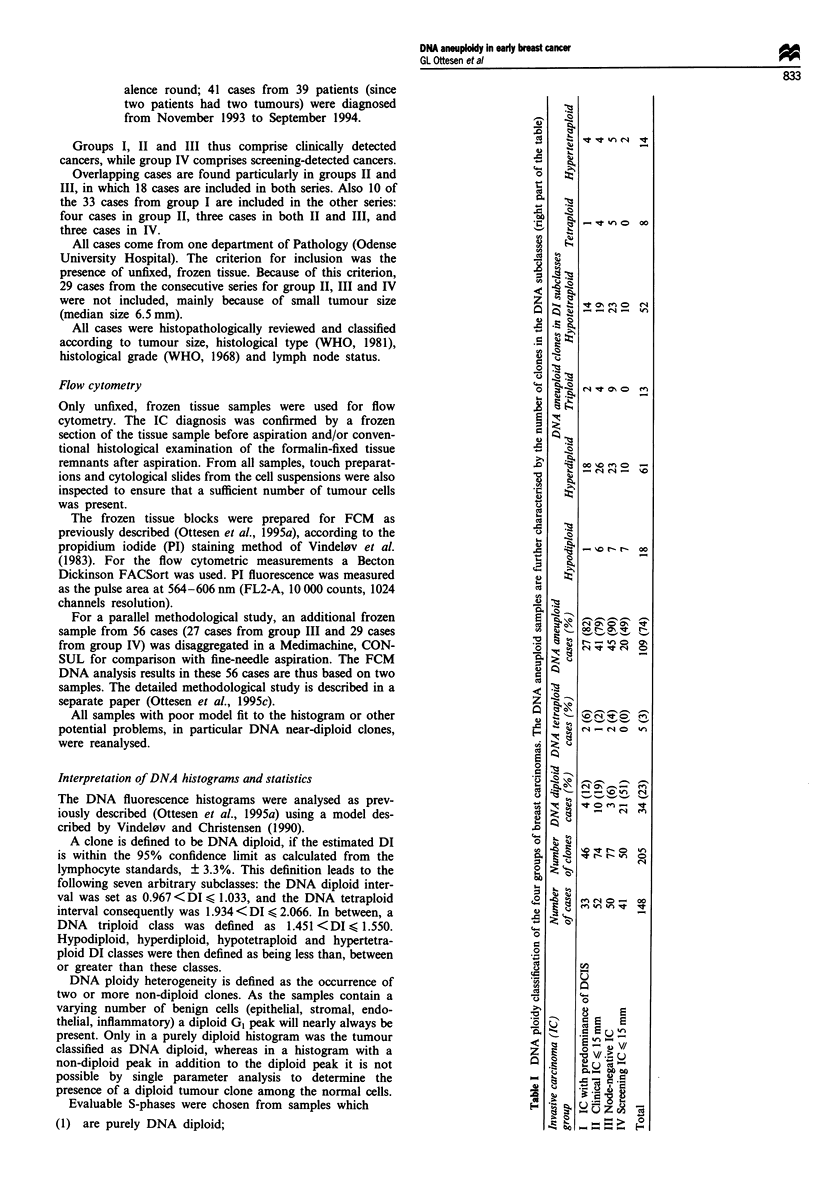
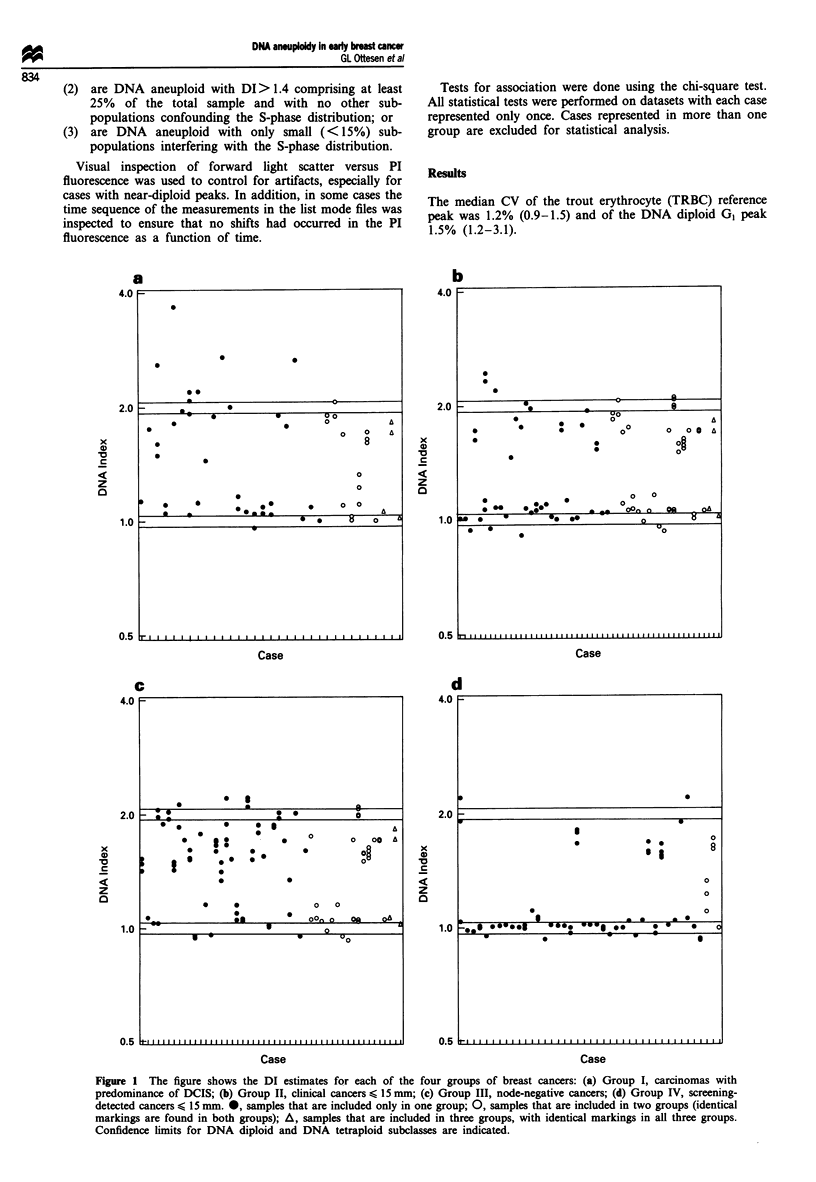
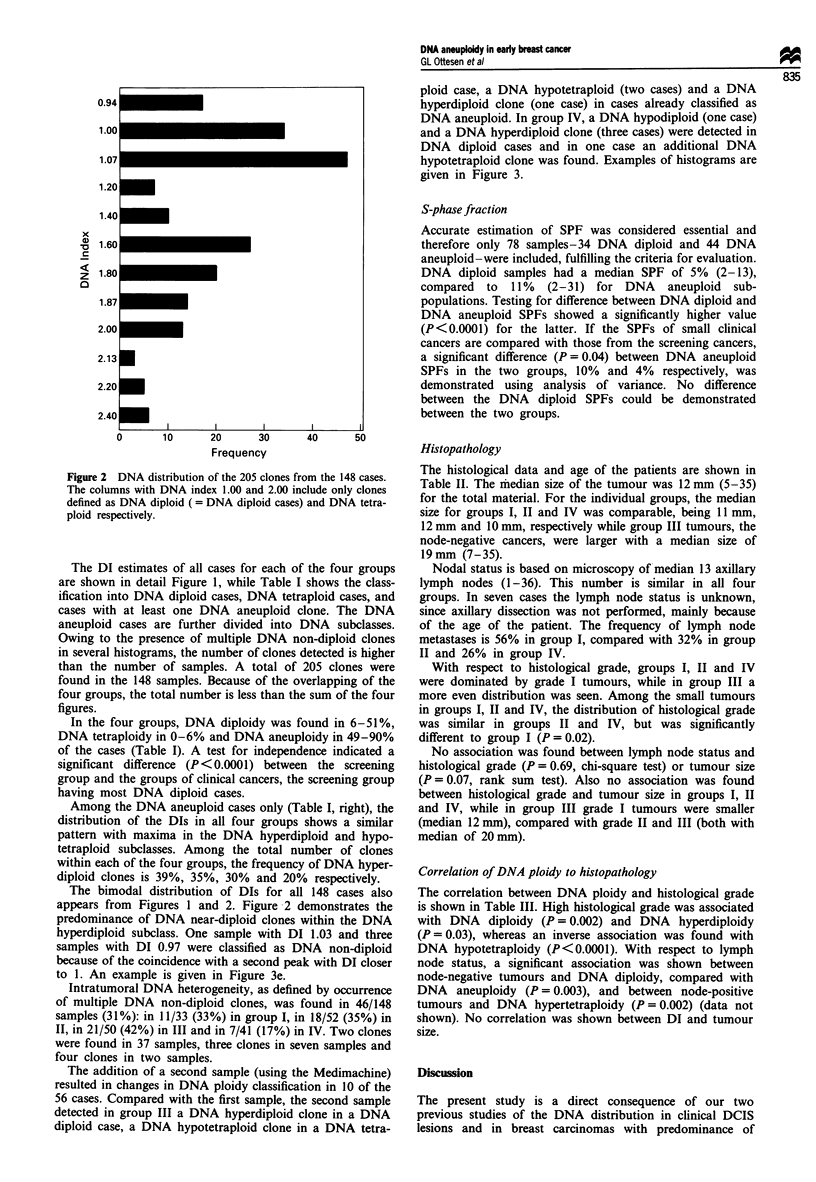
High-resolution flow cytometric (FCM) DNA analysis was performed on 148 unfixed, frozen tissue samples from four groups of early breast cancers: invasive carcinomas (ICs) with predominance of carcinoma in situ (DCIS) (group I), small clinical cancers < or = 15 mm (group II), node-negative, clinical cancers (group III) and small screening-detected cancers < or = 15 mm (group IV). The median tumour size was 12 mm. The aim of the study was to support, with a larger sample, our recent findings with respect to DNA ploidy pattern in the selected group of ICs with predominance of DCIS (group I). Similar results to this group were found for both the small clinical cancers and the node-negative cancers, with respect to frequency of DNA aneuploidy (79% and 90%), DNA index (DI) distribution, intratumoral DNA heterogeneity and S-phase fraction. A high frequency of DNA hyperdiploid clones was found, in particular related to highly differentiated tumours. A significant difference was found compared with the screening-detected cancers, which were characterised by a much lower frequency of DNA aneuploid samples (49%) and may represent a biologically specific group of low-malignant, slowly growing tumours. Associations were shown between histological grade and DI subclasses, and between lymph node status and DNA diploidy/aneuploidy, whereas DI was not correlated with tumour size. The DNA ploidy findings in this series of early cancers are concordant to our own results from preinvasive lesions as well as those reported from series of more advanced cancers.
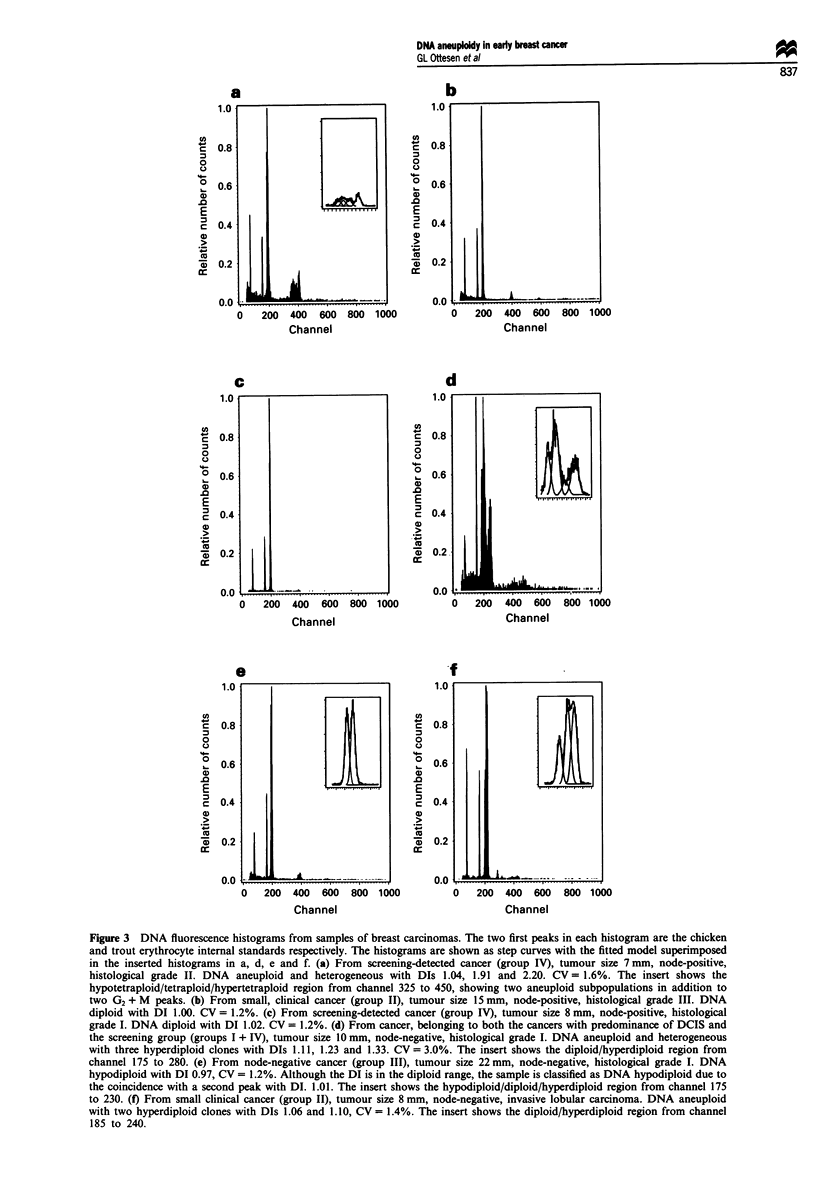
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alanen K. A., Klemi P. J., Joensuu H., Kujari H., Pekkala E. Comparison of fresh, ethanol-preserved, and paraffin-embedded samples in DNA flow cytometry. Cytometry. 1989 Jan;10(1):81–85. doi: 10.1002/cyto.990100114. [DOI] [PubMed] [Google Scholar]
- Balslev I., Christensen I. J., Rasmussen B. B., Larsen J. K., Lykkesfeldt A. E., Thorpe S. M., Rose C., Briand P., Mouridsen H. T. Flow cytometric DNA ploidy defines patients with poor prognosis in node-negative breast cancer. Int J Cancer. 1994 Jan 2;56(1):16–25. doi: 10.1002/ijc.2910560105. [DOI] [PubMed] [Google Scholar]
- Beerman H., Kluin P. M., Hermans J., van de Velde C. J., Cornelisse C. J. Prognostic significance of DNA-ploidy in a series of 690 primary breast cancer patients. Int J Cancer. 1990 Jan 15;45(1):34–39. doi: 10.1002/ijc.2910450108. [DOI] [PubMed] [Google Scholar]
- Beerman H., Smit V. T., Kluin P. M., Bonsing B. A., Hermans J., Cornelisse C. J. Flow cytometric analysis of DNA stemline heterogeneity in primary and metastatic breast cancer. Cytometry. 1991;12(2):147–154. doi: 10.1002/cyto.990120208. [DOI] [PubMed] [Google Scholar]
- Cornelisse C. J., Tanke H. J., de Koning H., de la Riviere G. B. DNA ploidy analysis and cytologic examination of sorted cell populations from human breast tumors. Anal Quant Cytol. 1983 Sep;5(3):173–183. [PubMed] [Google Scholar]
- Ewers S. B., Baldetorp B., Killander D., Långström E. Flow cytometry DNA ploidy and number of cell populations in the primary breast cancer and their correlation to the prognosis. Acta Oncol. 1989;28(6):913–918. doi: 10.3109/02841868909092331. [DOI] [PubMed] [Google Scholar]
- Fernö M., Baldetorp B., Borg A., Olsson H., Sigurdsson H., Killander D. Flow cytometric DNA index and S-phase fraction in breast cancer in relation to other prognostic variables and to clinical outcome. Acta Oncol. 1992;31(2):157–165. doi: 10.3109/02841869209088897. [DOI] [PubMed] [Google Scholar]
- Frierson H. F., Jr Flow cytometric analysis of ploidy in solid neoplasms: comparison of fresh tissues with formalin-fixed paraffin-embedded specimens. Hum Pathol. 1988 Mar;19(3):290–294. doi: 10.1016/s0046-8177(88)80521-5. [DOI] [PubMed] [Google Scholar]
- Hatschek T., Fagerberg G., Stål O., Sullivan S., Carstensen J., Gröntoft O., Nordenskjöld B. Cytometric characterization and clinical course of breast cancer diagnosed in a population-based screening program. Cancer. 1989 Sep 1;64(5):1074–1081. doi: 10.1002/1097-0142(19890901)64:5<1074::aid-cncr2820640519>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Hedley D. W. DNA Cytometry Consensus Conference. DNA flow cytometry and breast cancer. Breast Cancer Res Treat. 1993 Oct;28(1):51–53. doi: 10.1007/BF00666356. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P. Comparison of fresh and paraffin-embedded tissue as starting material for DNA flow cytometry and evaluation of intratumor heterogeneity. Cytometry. 1988 Mar;9(2):164–169. doi: 10.1002/cyto.990090211. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Kärkkäinen A., Auvinen O., Mattila J., Koivula T., Hakama M. DNA flow cytometric analysis indicates that many breast cancers detected in the first round of mammographic screening have a low malignant potential. Int J Cancer. 1988 Nov 15;42(5):697–702. doi: 10.1002/ijc.2910420511. [DOI] [PubMed] [Google Scholar]
- Ottesen G. L., Christensen I. J., Larsen J. K., Christiansen J., Hansen B., Andersen J. A. DNA analysis of in situ ductal carcinoma of the breast via flow cytometry. Cytometry. 1995 Sep 15;22(3):168–176. doi: 10.1002/cyto.990220303. [DOI] [PubMed] [Google Scholar]
- Ottesen G. L., Christensen I. J., Larsen J. K., Hansen B., Andersen A. J. Flow cytometric DNA analysis of breast cancers with predominance of carcinoma in situ: a comparison of the premalignant and malignant components. Clin Cancer Res. 1995 Aug;1(8):881–888. [PubMed] [Google Scholar]
- Pandis N., Jin Y., Gorunova L., Petersson C., Bardi G., Idvall I., Johansson B., Ingvar C., Mandahl N., Mitelman F. Chromosome analysis of 97 primary breast carcinomas: identification of eight karyotypic subgroups. Genes Chromosomes Cancer. 1995 Mar;12(3):173–185. doi: 10.1002/gcc.2870120304. [DOI] [PubMed] [Google Scholar]
- Stål O., Brisfors A., Carstensen J., Ferraud L., Hatschek T., Nordenskjöld B. Interrelations between cellular DNA content, S-phase fraction, hormone receptor status and age in primary breast cancer. A series of 1,342 consecutively detected tumors. South-East Sweden Breast Cancer Group. Acta Oncol. 1992;31(3):283–292. doi: 10.3109/02841869209108174. [DOI] [PubMed] [Google Scholar]
- Uyterlinde A. M., Baak J. P., Schipper N. W., Peterse H. J., Meijer J. W., Vooys P. G., Matze E. Prognostic value of morphometry and DNA flow-cytometry features of invasive breast cancers detected by population screening: comparison with control group of hospital patients. Int J Cancer. 1991 May 10;48(2):173–181. doi: 10.1002/ijc.2910480204. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry. 1990;11(7):753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Zalupski M. M., Maciorowski Z., Ryan J. R., Ensley J. F., Hussein M. E., Sundareson A. S., Baker L. H. DNA content parameters of paraffin-embedded soft tissue sarcomas: optimization of retrieval technique and comparison to fresh tissue. Cytometry. 1993;14(3):327–333. doi: 10.1002/cyto.990140313. [DOI] [PubMed] [Google Scholar]



