Abstract
This study examined the effect of transforming growth factor beta-1 (TGF-beta 1) on c-myc, RB1, junB and p53 expression together with pRb phosphorylation, in carcinoma-derived and normal human oral keratinocytes with a range of inhibitory responses to this ligand. Amplification of c-myc was observed in eight of eight tumour-derived cell lines and resulted in corresponding mRNA expression. The down-regulation of c-myc expression by TGF-beta 1 predominantly reflected growth inhibition by TGF-beta 1, but in two of eight tumour-derived cell lines which were partially responsive to TGF-beta 1 c-myc expression was unaltered by this ligand. While RB1 mRNA levels were unaltered by TGF-beta 1, the ligand caused the accumulation of the underphosphorylated form of the Rb protein in all cells irrespective of TGF-beta 1-induced growth arrest. junB expression was up-regulated by TGF-beta 1 in cells with a range of growth inhibitory responses. All cells contained mutant p53. TGF-beta 1 did not affect p53 mRNA expression in both tumour-derived and normal keratinocytes and there was no alteration in p53 protein levels in keratinocytes expressing stable p53 protein following TGF-beta 1 treatment. The data indicate that TGF-beta-induced growth control can exist independently of the presence of mutant p53 and the control of Rb phosphorylation and c-myc down-regulation. It may be that TGF-beta growth inhibition occurs via multiple mechanisms and that the loss of one pathway during tumour progression does not necessarily result in the abrogation of TGF-beta-induced growth control.
Full text
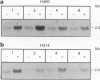
PDF

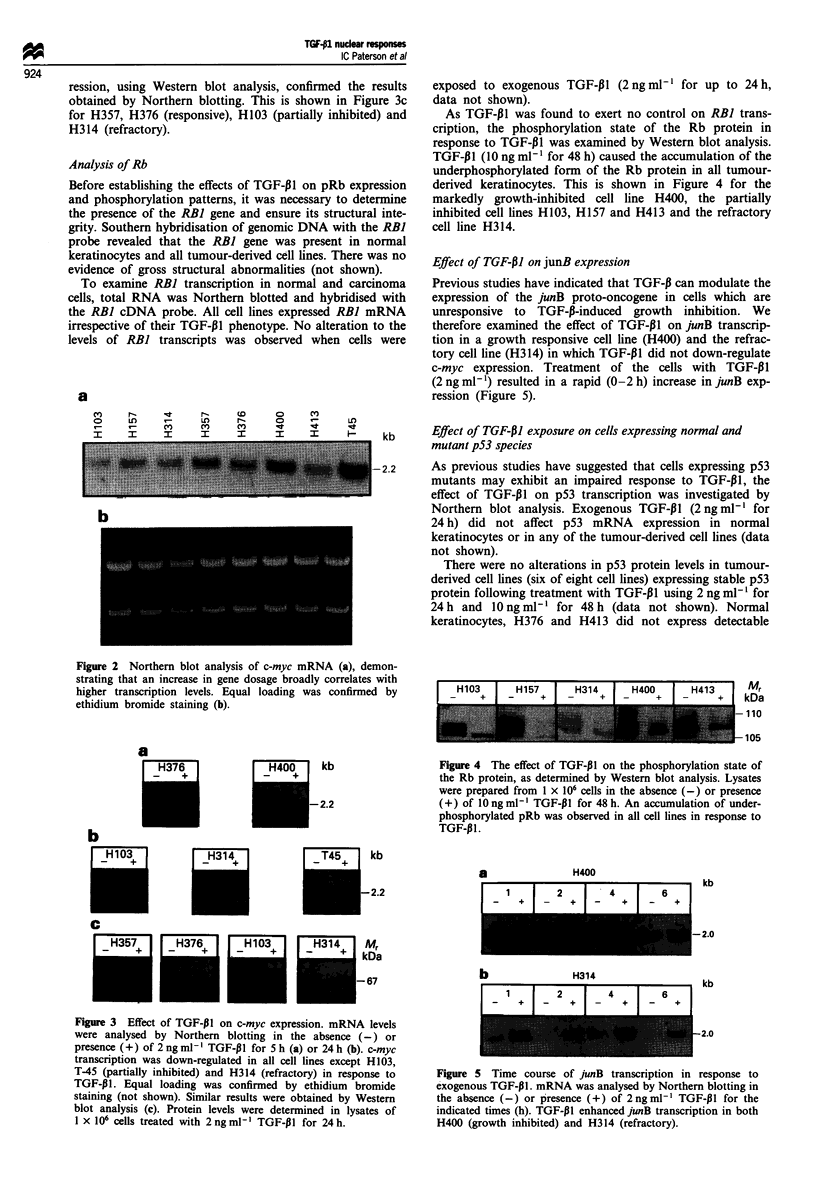



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Koskinen P., Mäkelä T. P., Saksela K., Sistonen L., Winqvist R. myc oncogenes: activation and amplification. Biochim Biophys Acta. 1987 Apr 20;907(1):1–32. doi: 10.1016/0304-419x(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Ebner R., Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-beta activities. Science. 1993 May 28;260(5112):1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Bascom C. C., Sipes N. J., Graves-Deal R., Weissman B. E., Moses H. L. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988 Aug;8(8):3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Oliver C. J., Sluss H. K., Miller S. J., Peeper D. S. p53-dependent repression of CDK4 translation in TGF-beta-induced G1 cell-cycle arrest. Genes Dev. 1995 Jan 15;9(2):204–217. doi: 10.1101/gad.9.2.204. [DOI] [PubMed] [Google Scholar]
- Fynan T. M., Reiss M. Resistance to inhibition of cell growth by transforming growth factor-beta and its role in oncogenesis. Crit Rev Oncog. 1993;4(5):493–540. [PubMed] [Google Scholar]
- Game S. M., Huelsen A., Patel V., Donnelly M., Yeudall W. A., Stone A., Fusenig N. E., Prime S. S. Progressive abrogation of TGF-beta 1 and EGF growth control is associated with tumour progression in ras-transfected human keratinocytes. Int J Cancer. 1992 Sep 30;52(3):461–470. doi: 10.1002/ijc.2910520322. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Spillare E., Forrester K., Lehman T. A., Kispert J., Welsh J. A., Pfeifer A. M., Lechner J. F., Baker S. J., Vogelstein B. Mutant p53 can induce tumorigenic conversion of human bronchial epithelial cells and reduce their responsiveness to a negative growth factor, transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2759–2763. doi: 10.1073/pnas.89.7.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe P. H., Dobrowolski S. F., Reddy K. B., Stacey D. W. Release from G1 growth arrest by transforming growth factor beta 1 requires cellular ras activity. J Biol Chem. 1993 Oct 5;268(28):21448–21452. [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Wang X. F., Weinberg R. A., Cheifetz S., Massagué J. Absence of TGF-beta receptors and growth inhibitory responses in retinoblastoma cells. Science. 1988 Apr 8;240(4849):196–199. doi: 10.1126/science.2895499. [DOI] [PubMed] [Google Scholar]
- Koike M., Ishino K., Huh N., Kuroki T. Growth inhibition of SV40-transformed human keratinocytes by TGF-beta s is not linked to dephosphorylation of the Rb gene product. Biochem Biophys Res Commun. 1994 Jun 15;201(2):673–681. doi: 10.1006/bbrc.1994.1753. [DOI] [PubMed] [Google Scholar]
- Krieg P., Amtmann E., Sauer G. The simultaneous extraction of high-molecular-weight DNA and of RNA from solid tumors. Anal Biochem. 1983 Oct 15;134(2):288–294. doi: 10.1016/0003-2697(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Laiho M., Weis F. M., Boyd F. T., Ignotz R. A., Massagué J. Responsiveness to transforming growth factor-beta (TGF-beta) restored by genetic complementation between cells defective in TGF-beta receptors I and II. J Biol Chem. 1991 May 15;266(14):9108–9112. [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990 Oct 25;265(30):18518–18524. [PubMed] [Google Scholar]
- Landesman Y., Pagano M., Draetta G., Rotter V., Fusenig N. E., Kimchi A. Modifications of cell cycle controlling nuclear proteins by transforming growth factor beta in the HaCaT keratinocyte cell line. Oncogene. 1992 Aug;7(8):1661–1665. [PubMed] [Google Scholar]
- Manning A. M., Williams A. C., Game S. M., Paraskeva C. Differential sensitivity of human colonic adenoma and carcinoma cells to transforming growth factor beta (TGF-beta): conversion of an adenoma cell line to a tumorigenic phenotype is accompanied by a reduced response to the inhibitory effects of TGF-beta. Oncogene. 1991 Aug;6(8):1471–1476. [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Matlashewski G. J., Tuck S., Pim D., Lamb P., Schneider J., Crawford L. V. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol. 1987 Feb;7(2):961–963. doi: 10.1128/mcb.7.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C., Ramon y Cajal S., Dotto G. P. Escape from transforming growth factor beta control and oncogene cooperation in skin tumor development. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9613–9617. doi: 10.1073/pnas.88.21.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Pietenpol J. A., Pittelkow M. R., Holt J. T., Moses H. L. Transforming growth factor beta 1 regulation of c-myc expression, pRB phosphorylation, and cell cycle progression in keratinocytes. Cell Growth Differ. 1992 May;3(5):291–298. [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Patel V., Yeudall W. A., Gardner A., Mutlu S., Scully C., Prime S. S. Consistent chromosomal anomalies in keratinocyte cell lines derived from untreated malignant lesions of the oral cavity. Genes Chromosomes Cancer. 1993 Jun;7(2):109–115. doi: 10.1002/gcc.2870070208. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Holt J. T., Stein R. W., Moses H. L. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci U S A. 1990 May;87(10):3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Prime S. S., Matthews J. B., Patel V., Game S. M., Donnelly M., Stone A., Paterson I. C., Sandy J. R., Yeudall W. A. TGF-beta receptor regulation mediates the response to exogenous ligand but is independent of the degree of cellular differentiation in human oral keratinocytes. Int J Cancer. 1994 Feb 1;56(3):406–412. doi: 10.1002/ijc.2910560320. [DOI] [PubMed] [Google Scholar]
- Prime S. S., Nixon S. V., Crane I. J., Stone A., Matthews J. B., Maitland N. J., Remnant L., Powell S. K., Game S. M., Scully C. The behaviour of human oral squamous cell carcinoma in cell culture. J Pathol. 1990 Mar;160(3):259–269. doi: 10.1002/path.1711600313. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Rosen D. M., Seyedin S. M. Binding of transforming growth factor-beta to cell surface proteins varies with cell type. Mol Endocrinol. 1989 Feb;3(2):261–272. doi: 10.1210/mend-3-2-261. [DOI] [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Wieser R., Attisano L., Wrana J. L., Massagué J. Signaling activity of transforming growth factor beta type II receptors lacking specific domains in the cytoplasmic region. Mol Cell Biol. 1993 Dec;13(12):7239–7247. doi: 10.1128/mcb.13.12.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Yan Z., Winawer S., Friedman E. Two different signal transduction pathways can be activated by transforming growth factor beta 1 in epithelial cells. J Biol Chem. 1994 May 6;269(18):13231–13237. [PubMed] [Google Scholar]
- Yeudall W. A., Paterson I. C., Patel V., Prime S. S. Presence of human papillomavirus sequences in tumour-derived human oral keratinocytes expressing mutant p53. Eur J Cancer B Oral Oncol. 1995 Mar;31B(2):136–143. doi: 10.1016/0964-1955(94)00030-8. [DOI] [PubMed] [Google Scholar]
- Zentella A., Weis F. M., Ralph D. A., Laiho M., Massagué J. Early gene responses to transforming growth factor-beta in cells lacking growth-suppressive RB function. Mol Cell Biol. 1991 Oct;11(10):4952–4958. doi: 10.1128/mcb.11.10.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]