Abstract
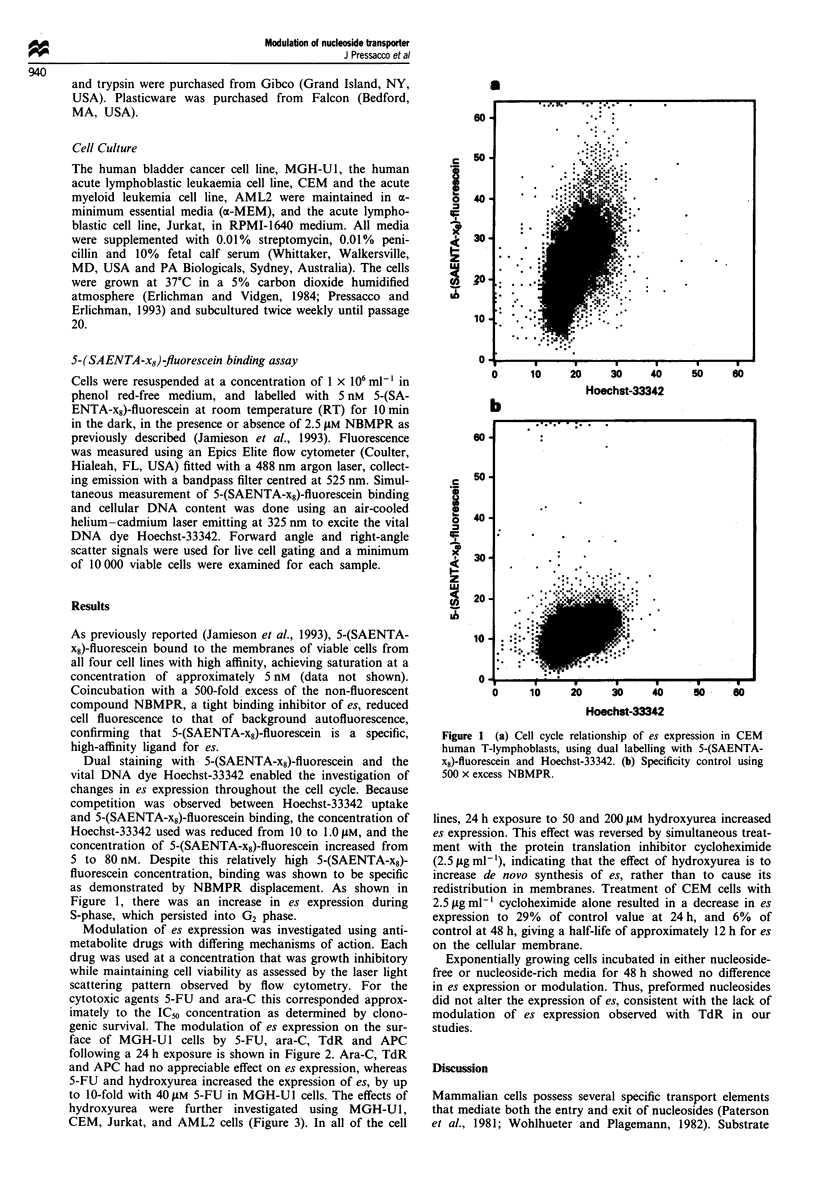
Expression of the equilibrative, S-(p-nitrobenzyl)-6-thioinosine (NBMPR)-sensitive nucleoside transporter (es), a component of the nucleoside salvage pathway, was measured during unperturbed growth and following exposure to various antimetabolites at growth-inhibitory concentrations. The probe 5-(SAENTA-x8)-fluorescein is a highly modified form of adenosine incorporating a fluorescein molecule. It binds. with high affinity and specificity to the (es) nucleoside transporter at a 1:1 stoichiometry, allowing reliable estimates of es expression by flow cytometry. Using a dual labelling technique which combined the vital DNA dye Hoechst-33342 and 5-(SAENTA-x8)-fluorescein, we found that surface expression of es approximately doubled between G1 and G2 + M phases of the cell cycle. To address the question of whether es expression could be modulated in cells exposed to drugs which inhibit de novo synthesis of nucleotides, cells were exposed to antimetabolite drugs having different modes of action. Hydroxyurea and 5-fluorouracil (5-FU), which inhibit the de novo synthesis of DNA precursors, produced increases in the expression of es. In contrast, cytosine arabinoside (ara-C) and aphidicolin, which directly inhibit DNA synthesis, produced no significant increase in es expression. Thymidine (TdR), which is an allosteric inhibitor of ribonucleotide reductase that depletes dATP, dCTP and dGTP pools while repleting the dTTP pool, had no significant effect on es expression. These data suggest that surface expression of the es nucleoside transporter is regulated by a mechanism which is sensitive to the supply of deoxynucleotides. Because 5-FU (which specifically depletes dTTP pools) causes a large increase in expression whereas TdR (which depletes all precursors except dTTP) does not, this mechanism might be particularly sensitive to dTTP pools.
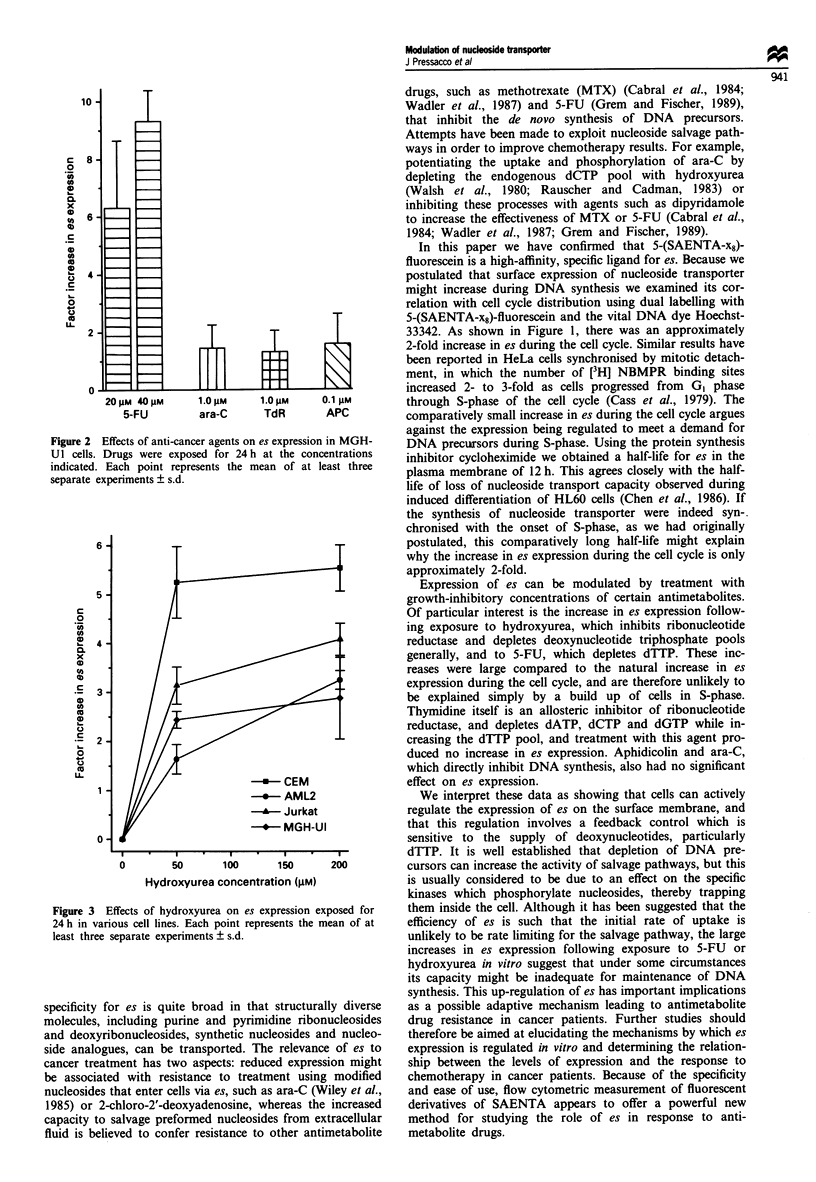
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agbanyo F. R., Vijayalakshmi D., Craik J. D., Gati W. P., McAdam D. P., Asakura J., Robins M. J., Paterson A. R., Cass C. E. 5'-S-(2-aminoethyl)-N6-(4-nitrobenzyl)-5'-thioadenosine (SAENTA), a novel ligand with high affinity for polypeptides associated with nucleoside transport. Partial purification of the nitrobenzylthioinosine-binding protein of pig erythrocytes by affinity chromatography. Biochem J. 1990 Sep 15;270(3):605–614. doi: 10.1042/bj2700605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt J. A. Heterogeneity of nucleoside transport in mammalian cells. Two types of transport activity in L1210 and other cultured neoplastic cells. Mol Pharmacol. 1983 Nov;24(3):479–484. [PubMed] [Google Scholar]
- Belt J. A., Marina N. M., Phelps D. A., Crawford C. R. Nucleoside transport in normal and neoplastic cells. Adv Enzyme Regul. 1993;33:235–252. doi: 10.1016/0065-2571(93)90021-5. [DOI] [PubMed] [Google Scholar]
- Cabral S., Leis S., Bover L., Nembrot M., Mordoh J. Dipyridamole inhibits reversion by thymidine of methotrexate effect and increases drug uptake in Sarcoma 180 cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3200–3203. doi: 10.1073/pnas.81.10.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass C. E., Dahlig E., Lau E. Y., Lynch T. P., Paterson A. R. Fluctuations in nucleoside uptake and binding of the inhibitor of nucleoside transport, nitrobenzylthioinosine, during the replication cycle of HeLa cells. Cancer Res. 1979 Apr;39(4):1245–1252. [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Chen S. F., Cleaveland J. S., Hollmann A. B., Wiemann M. C., Parks R. E., Jr, Stoeckler J. D. Changes in nucleoside transport of HL-60 human promyelocytic cells during N,N-dimethylformamide induced differentiation. Cancer Res. 1986 Jul;46(7):3449–3455. [PubMed] [Google Scholar]
- Crawford C. R., Belt J. A. Sodium-dependent, concentrative nucleoside transport in Walker 256 rat carcinosarcoma cells. Biochem Biophys Res Commun. 1991 Mar 29;175(3):846–851. doi: 10.1016/0006-291x(91)91642-p. [DOI] [PubMed] [Google Scholar]
- Erlichman C., Vidgen D. Cytotoxicity of adriamycin in MGH-U1 cells grown as monolayer cultures, spheroids, and xenografts in immune-deprived mice. Cancer Res. 1984 Nov;44(11):5369–5375. [PubMed] [Google Scholar]
- Grem J. L., Fischer P. H. Enhancement of 5-fluorouracil's anticancer activity by dipyridamole. Pharmacol Ther. 1989;40(3):349–371. doi: 10.1016/0163-7258(89)90084-3. [DOI] [PubMed] [Google Scholar]
- Jamieson G. P., Brocklebank A. M., Snook M. B., Sawyer W. H., Buolamwini J. K., Paterson A. R., Wiley J. S. Flow cytometric quantitation of nucleoside transporter sites on human leukemic cells. Cytometry. 1993;14(1):32–38. doi: 10.1002/cyto.990140107. [DOI] [PubMed] [Google Scholar]
- Nottebrock H., Then R. Thymidine concentrations in serum and urine of different animal species and man. Biochem Pharmacol. 1977 Nov 15;26(22):2175–2179. doi: 10.1016/0006-2952(77)90271-4. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Kolassa N., Cass C. E. Transport of nucleoside drugs in animal cells. Pharmacol Ther. 1981;12(3):515–536. doi: 10.1016/0163-7258(81)90096-6. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M., Woffendin C. Nucleoside and nucleobase transport in animal cells. Biochim Biophys Acta. 1988 Oct 11;947(3):405–443. doi: 10.1016/0304-4157(88)90002-0. [DOI] [PubMed] [Google Scholar]
- Pressacco J., Erlichman C. Combination studies with 3'-azido-3'-deoxythymidine (AZT) plus ICI D1694. Cytotoxic and biochemical effects. Biochem Pharmacol. 1993 Dec 3;46(11):1989–1997. doi: 10.1016/0006-2952(93)90641-9. [DOI] [PubMed] [Google Scholar]
- Rauscher F., 3rd, Cadman E. Biochemical and cytokinetic modulation of L1210 and HL-60 cells by hydroxyurea and effect on 1-beta-D-arabinofuranosylcytosine metabolism and cytotoxicity. Cancer Res. 1983 Jun;43(6):2688–2693. [PubMed] [Google Scholar]
- Roden M., Paterson A. R., Turnheim K. Sodium-dependent nucleoside transport in rabbit intestinal epithelium. Gastroenterology. 1991 Jun;100(6):1553–1562. doi: 10.1016/0016-5085(91)90652-2. [DOI] [PubMed] [Google Scholar]
- Wadler S., Subar M., Green M. D., Wiernik P. H., Muggia F. M. Phase II trial of oral methotrexate and dipyridamole in colorectal carcinoma. Cancer Treat Rep. 1987 Sep;71(9):821–824. [PubMed] [Google Scholar]
- Walsh C. T., Craig R. W., Agarwal R. P. Increased activation of 1-beta-D-arabinofuranosylcytosine by hydroxyurea in L1210 cells. Cancer Res. 1980 Sep;40(9):3286–3292. [PubMed] [Google Scholar]
- White J. C., Rathmell J. P., Capizzi R. L. Membrane transport influences the rate of accumulation of cytosine arabinoside in human leukemia cells. J Clin Invest. 1987 Feb;79(2):380–387. doi: 10.1172/JCI112823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Jones S. P., Sawyer W. H. Cytosine arabinoside transport by human leukaemic cells. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1067–1074. doi: 10.1016/0277-5379(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Taupin J., Jamieson G. P., Snook M., Sawyer W. H., Finch L. R. Cytosine arabinoside transport and metabolism in acute leukemias and T cell lymphoblastic lymphoma. J Clin Invest. 1985 Feb;75(2):632–642. doi: 10.1172/JCI111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlhueter R. M., Plagemann P. G. On the functional symmetry of nucleoside transport in mammalian cells. Biochim Biophys Acta. 1982 Jul 28;689(2):249–260. doi: 10.1016/0005-2736(82)90257-7. [DOI] [PubMed] [Google Scholar]


