Abstract
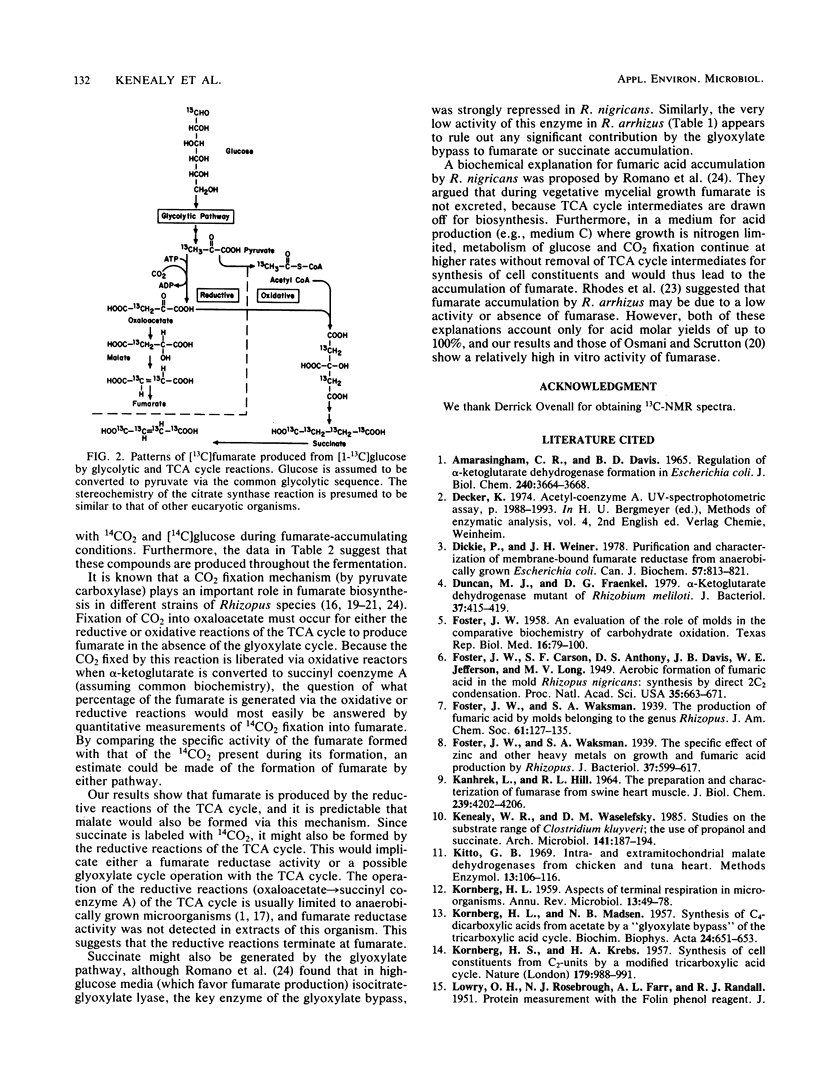
The accumulation and excretion of fumaric acid, and to a lesser extent malic and succinic acids, by Rhizopus arrhizus occurs under aerobic conditions in a high-glucose medium containing a limiting amount of nitrogen and a neutralizing agent (CaCO3). An overall four-carbon dicarboxylic acid molar yield of up to 145% (moles of acid produced per mole of glucose utilized) is obtained after incubation for 4 to 5 days. Evidence is presented that fumarate is synthesized from pyruvate via a carboxylation reaction yielding oxaloacetate, which is then converted to malate and further on to fumarate via the reductive reactions of the tricarboxylic acid cycle. The possible formation of fumarate from the normal (oxidative) operation of the tricarboxylic acid cycle was not excluded by the data. Yield, 13C nuclear magnetic resonance, and enzymatic activity studies were carried out in a strain of R. arrhizus which produces high levels of fumarate from glucose and carbonate. The observed high fumarate molar yield (greater than 100%) can therefore be explained in terms of the carboxylation of pyruvate and the operation of the reductive reactions of the tricarboxylic acid cycle under aerobic conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Fraenkel D. G. alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1979 Jan;137(1):415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W. An evaluation of the role of molds in the comparative biochemistry of carbohydrate oxidation. Tex Rep Biol Med. 1958;16(1):79–100. [PubMed] [Google Scholar]
- FOSTER J. W., CARSON S. F. Aerobic formation of fumaric acid in the mold Rhizopus nigricans, synthesis by direct C2 condensation. Proc Natl Acad Sci U S A. 1949 Dec;35(12):663–672. doi: 10.1073/pnas.35.12.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Waksman S. A. The Specific Effect of Zinc and Other Heavy Metals on the Growth and Nutrition of Rhizopus. J Bacteriol. 1939 Jun;37(6):599–617. doi: 10.1128/jb.37.6.599-617.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAREK L., HILL R. L. THE PREPARATION AND CHARACTERIZATION OF FUMARASE FROM SWINE HEART MUSCLE. J Biol Chem. 1964 Dec;239:4202–4206. [PubMed] [Google Scholar]
- KORNBERG H. L., KREBS H. A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature. 1957 May 18;179(4568):988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., MADSEN N. B. Synthesis of C4-dicarboxylic acids from acetate by a glyoxylate bypass of the tricarboxylic acid cycle. Biochim Biophys Acta. 1957 Jun;24(3):651–653. doi: 10.1016/0006-3002(57)90268-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGULIES M., VISHNIAC W. Dissimilation of glucose by the MX strain of Rhizopus. J Bacteriol. 1961 Jan;81:1–9. doi: 10.1128/jb.81.1.1-9.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON J. A. The purification and properties of yeast isocitric lyase. J Biol Chem. 1959 Jan;234(1):5–10. [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Scrutton M. C. The sub-cellular localisation and regulatory properties of pyruvate carboxylase from Rhizopus arrhizus. Eur J Biochem. 1985 Feb 15;147(1):119–128. doi: 10.1111/j.1432-1033.1985.tb08727.x. [DOI] [PubMed] [Google Scholar]
- Overman S. A., Romano A. H. Pyruvate carboxylase of Rhizopus nigricans and its role in fumaric acid production. Biochem Biophys Res Commun. 1969 Oct 22;37(3):457–463. doi: 10.1016/0006-291x(69)90937-1. [DOI] [PubMed] [Google Scholar]
- RHODES R. A., MOYER A. J., SMITH M. L., KELLEY S. E. Production of fumaric acid by Rhizopus arrhizus. Appl Microbiol. 1959 Mar;7(2):74–80. doi: 10.1128/am.7.2.74-80.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. A., Lagoda A. A., Misenheimer T. J., Smith M. L., Anderson R. F., Jackson R. W. Production of Fumaric Acid in 20-Liter Fermentors. Appl Microbiol. 1962 Jan;10(1):9–15. doi: 10.1128/am.10.1.9-15.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Bright M. M., Scott W. E. Mechanism of fumaric acid accumulation in Rhizopus nigricans. J Bacteriol. 1967 Feb;93(2):600–604. doi: 10.1128/jb.93.2.600-604.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem Anal. 1974;22:123–175. doi: 10.1002/9780470110423.ch3. [DOI] [PubMed] [Google Scholar]
- WEGENER W. S., ROMANO A. H. CONTROL OF ISOCITRATASE FORMATION IN RHIZOPUS NIGRICANS. J Bacteriol. 1964 Jan;87:156–161. doi: 10.1128/jb.87.1.156-161.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


