Abstract
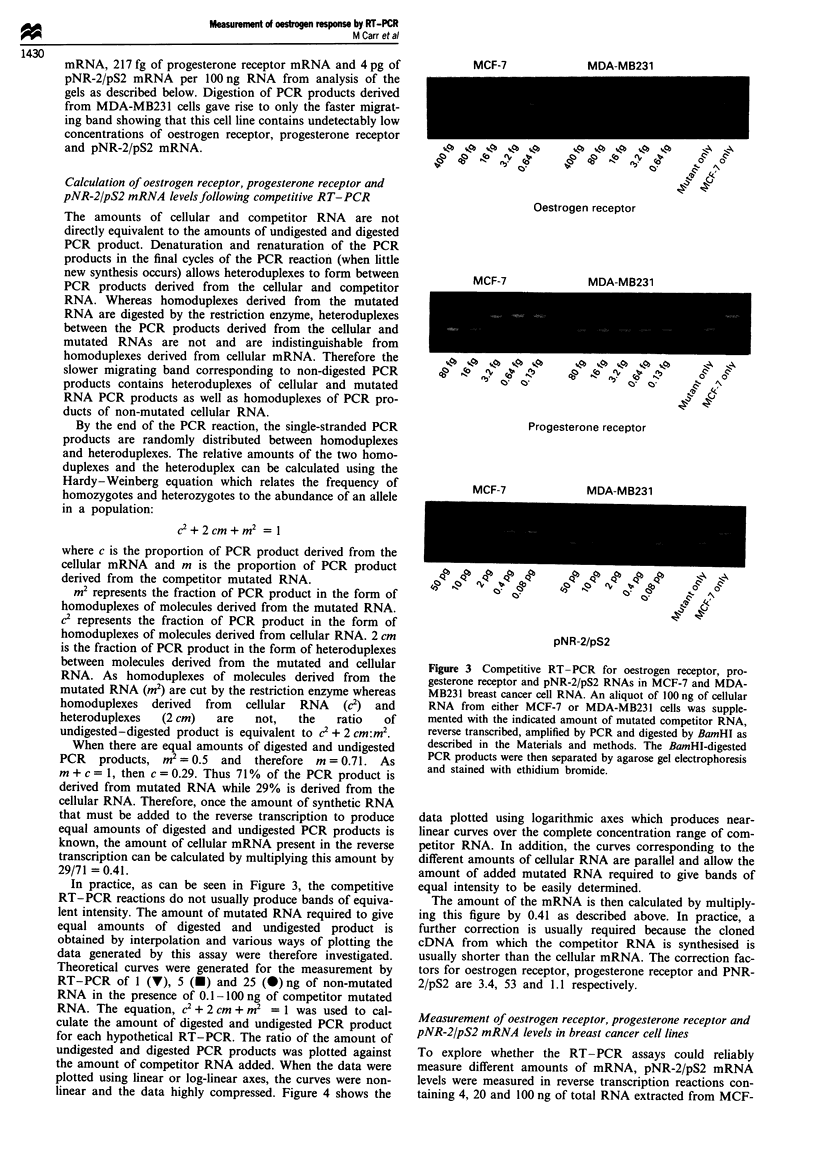
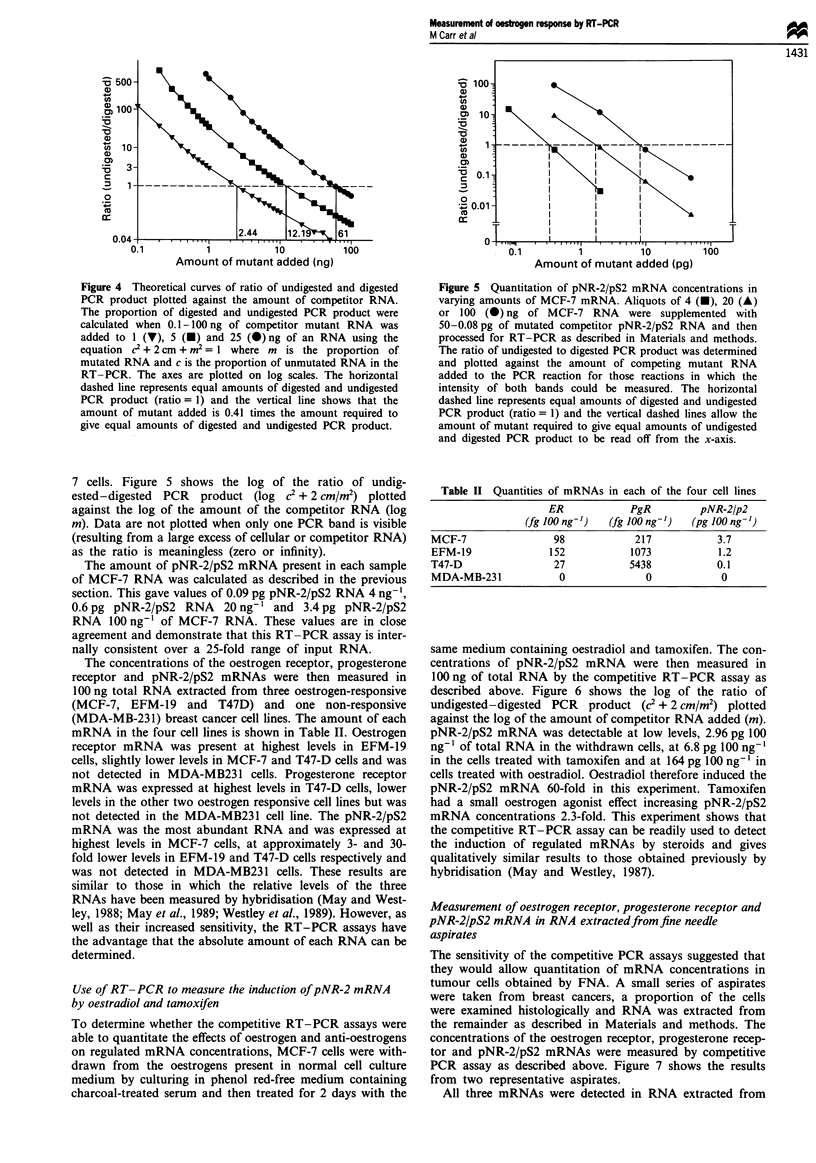
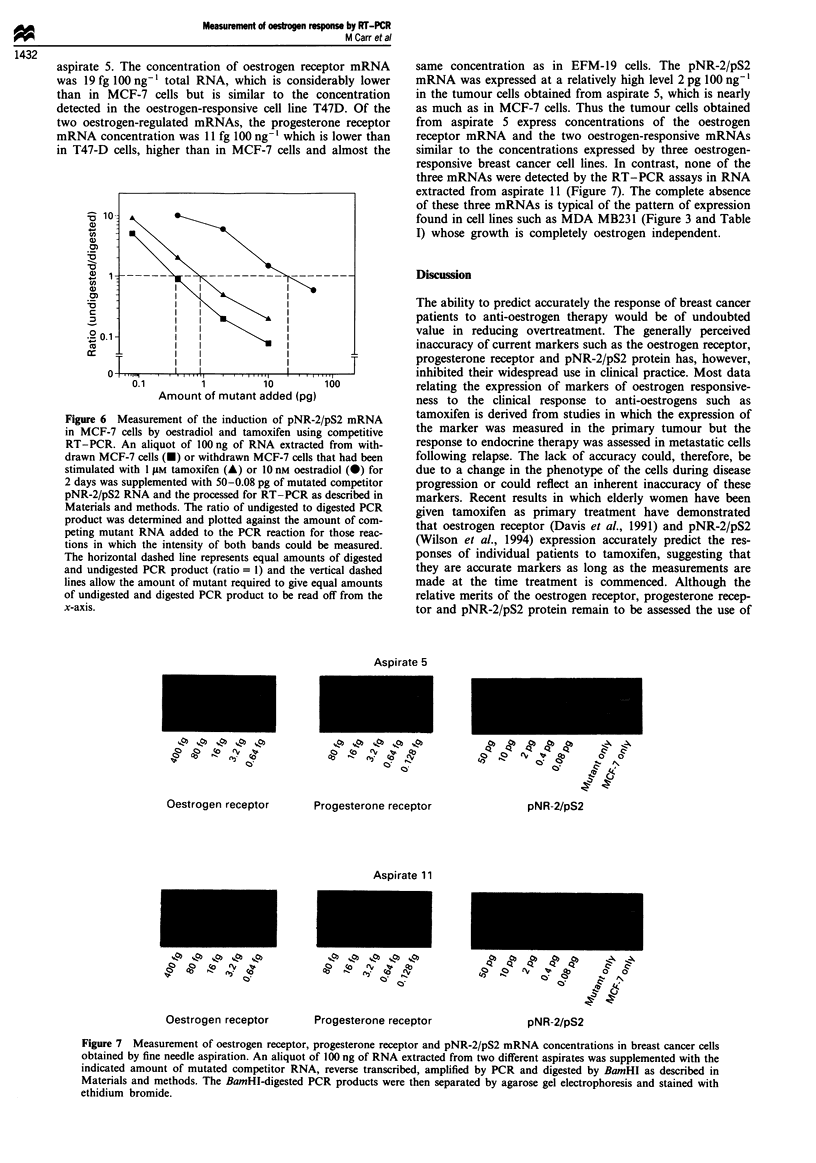
Competitive polymerase chain reaction assays have been developed for the quantitation of oestrogen receptor mRNA and two oestrogen-regulated mRNAs (progesterone receptor and pNR-2/pS2) in breast cancer cells. These assays are more sensitive than traditional hybridisation techniques, do not require the use of radioisotopes, measure absolute amounts of messenger RNAs and can be used to measure the expression of mRNAs in small numbers of tumour cells obtained by fine-needle aspiration (FNA). These assays should prove useful for predicting the hormone responsiveness of breast cancer from tumour cells obtained by FNA at diagnosis and could be particularly useful in the management of elderly/frail patients who receive primary tamoxifen, or in other patients for whom tumour tissue for standard biochemical measurements is not available.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolakos M. J., Schuermann W. H., Frampton M. W., Utell M. J., Willey J. C. Measurement of gene expression by multiplex competitive polymerase chain reaction. Anal Biochem. 1993 Sep;213(2):277–284. doi: 10.1006/abio.1993.1421. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clifford S. C., Thomas D. J., Neal D. E., Lunec J. Increased mdr1 gene transcript levels in high-grade carcinoma of the bladder determined by quantitative PCR-based assay. Br J Cancer. 1994 Apr;69(4):680–686. doi: 10.1038/bjc.1994.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes R. C., Powles T. J., Berger U., Wilson P., McClelland R. A., Gazet J. C., Trott P. A., Ford H. T. Prediction of endocrine response in breast cancer by immunocytochemical detection of oestrogen receptor in fine-needle aspirates. Lancet. 1987 Sep 26;2(8561):701–703. doi: 10.1016/s0140-6736(87)91071-3. [DOI] [PubMed] [Google Scholar]
- Coombes R. C., Powles T. J., Berger U., Wilson P., McClelland R. A., Gazet J. C., Trott P. A., Ford H. T. Prediction of endocrine response in breast cancer by immunocytochemical detection of oestrogen receptor in fine-needle aspirates. Lancet. 1987 Sep 26;2(8561):701–703. doi: 10.1016/s0140-6736(87)91071-3. [DOI] [PubMed] [Google Scholar]
- Crawford D. J., Lope-Pihie A., Cowan S., George W. D., Leake R. E. Pre-operative determination of oestrogen receptor status in breast cancer by immunocytochemical staining of fine needle aspirates. Br J Surg. 1985 Dec;72(12):991–993. doi: 10.1002/bjs.1800721220. [DOI] [PubMed] [Google Scholar]
- Dante R., Ribieras S., Baldassini S., Martin V., Benzerara O., Bouteille C., Brémond A., Frappart L., Rio M. C., Lasne Y. Expression of an estrogen-induced breast cancer-associated protein (pS2) in benign and malignant human ovarian cysts. Lab Invest. 1994 Aug;71(2):188–192. [PubMed] [Google Scholar]
- Davies N., Moir G., Carpenter R., Cuthbert A., Herbert A., Royle G. T., Taylor I. ERICA predicts response to tamoxifen in elderly women with breast cancer. Ann R Coll Surg Engl. 1991 Nov;73(6):361–363. [PMC free article] [PubMed] [Google Scholar]
- Flowers J. L., Burton G. V., Cox E. B., McCarty K. S., Sr, Dent G. A., Geisinger K. R., McCarty K. S., Jr Use of monoclonal antiestrogen receptor antibody to evaluate estrogen receptor content in fine needle aspiration breast biopsies. Ann Surg. 1986 Mar;203(3):250–254. doi: 10.1097/00000658-198603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua S. A., Falette N. F., McGuire W. L. Sensitive detection of estrogen receptor RNA by polymerase chain reaction assay. J Natl Cancer Inst. 1990 May 16;82(10):858–861. doi: 10.1093/jnci/82.10.858. [DOI] [PubMed] [Google Scholar]
- Gaskell D. J., Hawkins R. A., Sangsterl K., Chetty U., Forrest A. P. Relation between immunocytochemical estimation of oestrogen receptor in elderly patients with primary breast cancer and response to tamoxifen. Lancet. 1989 May 13;1(8646):1044–1046. doi: 10.1016/s0140-6736(89)92445-8. [DOI] [PubMed] [Google Scholar]
- Gazet J. C., Markopoulos C., Ford H. T., Coombes R. C., Bland J. M., Dixon R. C. Prospective randomised trial of tamoxifen versus surgery in elderly patients with breast cancer. Lancet. 1988 Mar 26;1(8587):679–681. doi: 10.1016/s0140-6736(88)91478-x. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Piggott N. H., Mallick U. K., Nicholson S., Farndon J. R., Westley B. R., May F. E. pNR-2/pS2 immunohistochemical staining in breast cancer: correlation with prognostic factors and endocrine response. Br J Cancer. 1991 Apr;63(4):615–622. doi: 10.1038/bjc.1991.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoof T., Riordan J. R., Tümmler B. Quantitation of mRNA by the kinetic polymerase chain reaction assay: a tool for monitoring P-glycoprotein gene expression. Anal Biochem. 1991 Jul;196(1):161–169. doi: 10.1016/0003-2697(91)90133-e. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. D., Westley B. R., May F. E. Oestrogenic activity of tamoxifen and its metabolites on gene regulation and cell proliferation in MCF-7 breast cancer cells. Br J Cancer. 1989 May;59(5):727–738. doi: 10.1038/bjc.1989.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Manni A. Endocrine therapy of metastatic breast cancer. J Endocrinol Invest. 1989 May;12(5):357–372. doi: 10.1007/BF03350007. [DOI] [PubMed] [Google Scholar]
- May F. E., Johnson M. D., Wiseman L. R., Wakeling A. E., Kastner P., Westley B. R. Regulation of progesterone receptor mRNA by oestradiol and antioestrogens in breast cancer cell lines. J Steroid Biochem. 1989 Dec;33(6):1035–1041. doi: 10.1016/0022-4731(89)90406-8. [DOI] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Cloning of estrogen-regulated messenger RNA sequences from human breast cancer cells. Cancer Res. 1986 Dec;46(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Effects of tamoxifen and 4-hydroxytamoxifen on the pNR-1 and pNR-2 estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1987 Nov 25;262(33):15894–15899. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Identification and characterization of estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1988 Sep 15;263(26):12901–12908. [PubMed] [Google Scholar]
- Osborne C. K., Yochmowitz M. G., Knight W. A., 3rd, McGuire W. L. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980 Dec 15;46(12 Suppl):2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Westley B. R., Hölzel F., May F. E. Effects of oestrogen and the antioestrogens, tamoxifen and LY117018, on four oestrogen-regulated RNAs in the EFM-19 breast cancer cell line. J Steroid Biochem. 1989 Mar;32(3):365–372. doi: 10.1016/0022-4731(89)90208-2. [DOI] [PubMed] [Google Scholar]
- Wilson Y. G., Rhodes M., Ibrahim N. B., Padfield C. J., Cawthorn S. J. Immunocytochemical staining of pS2 protein in fine-needle aspirate from breast cancer is an accurate guide to response to tamoxifen in patients aged over 70 years. Br J Surg. 1994 Aug;81(8):1155–1158. doi: 10.1002/bjs.1800810824. [DOI] [PubMed] [Google Scholar]
- Wundrack I., Müllenbach R., Welter C., Seitz G., Blin N. PCR expression analysis of the estrogen-inducible gene BCEI in gastrointestinal and other human tumors. Dis Markers. 1994 Oct;12(1):63–69. doi: 10.1155/1994/542396. [DOI] [PubMed] [Google Scholar]