Abstract
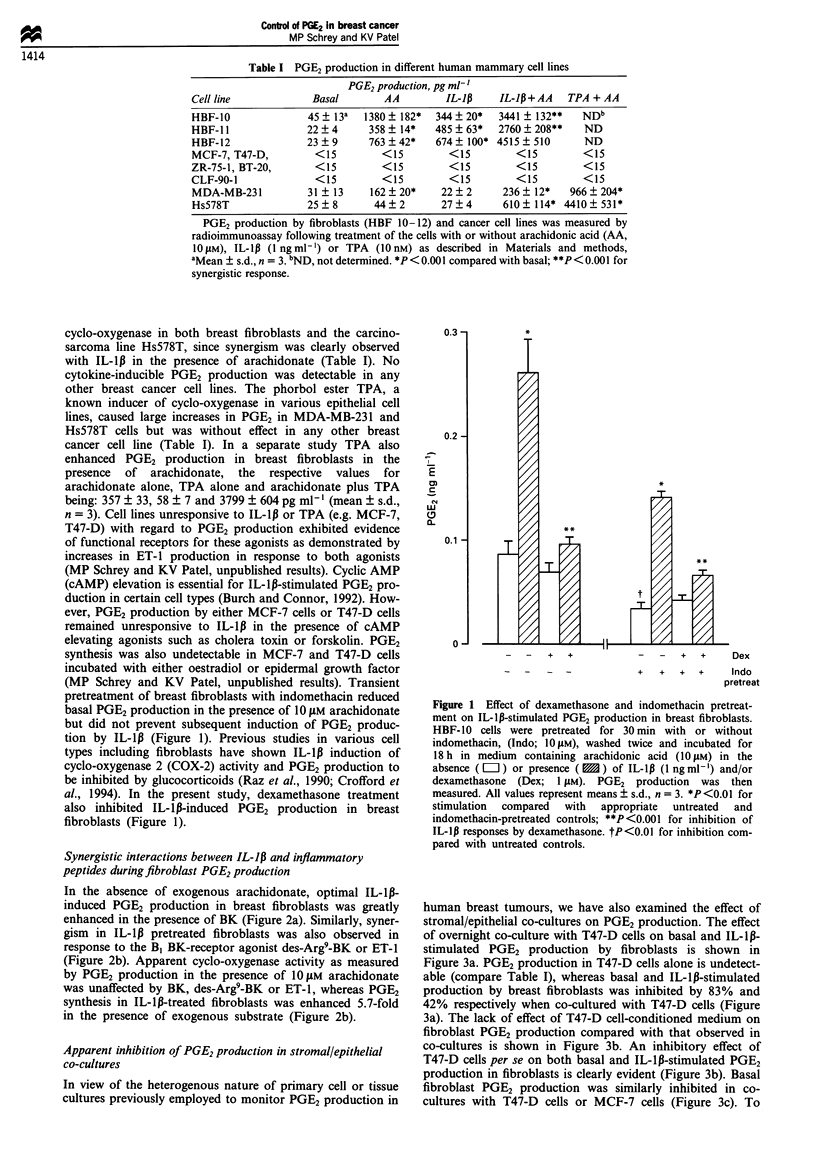
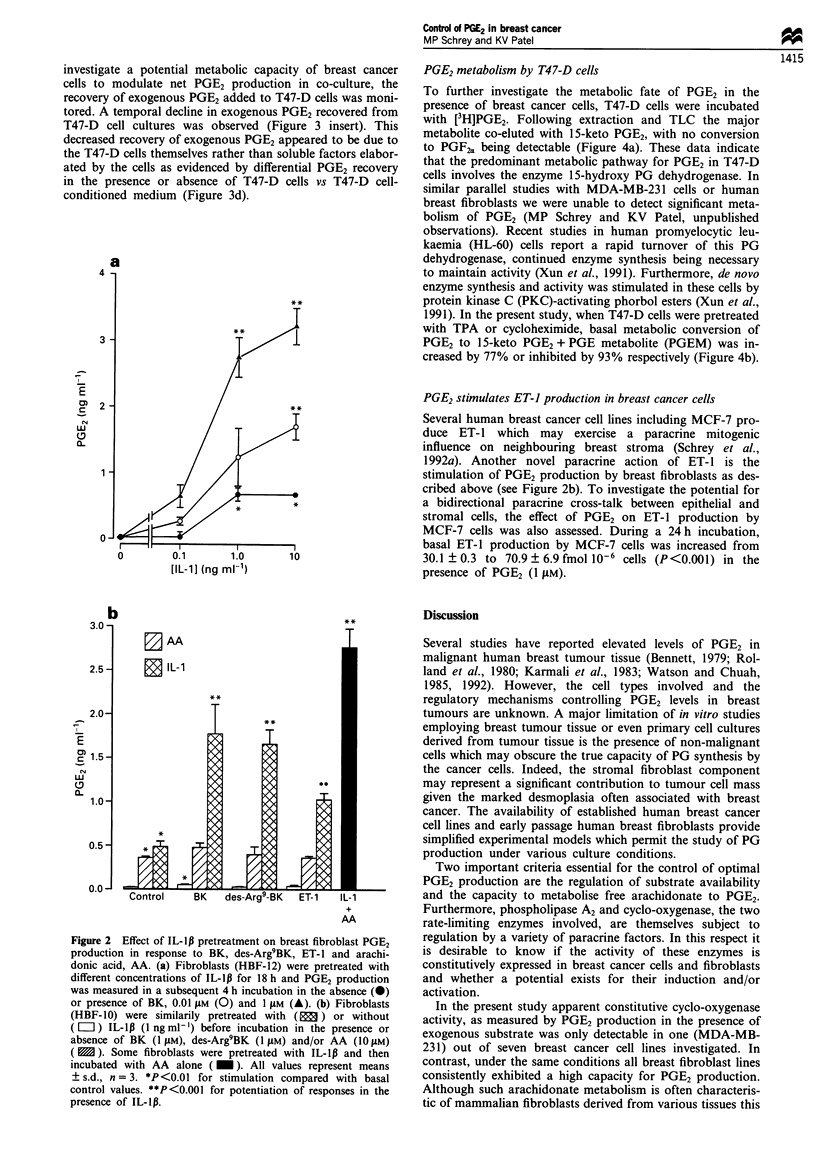
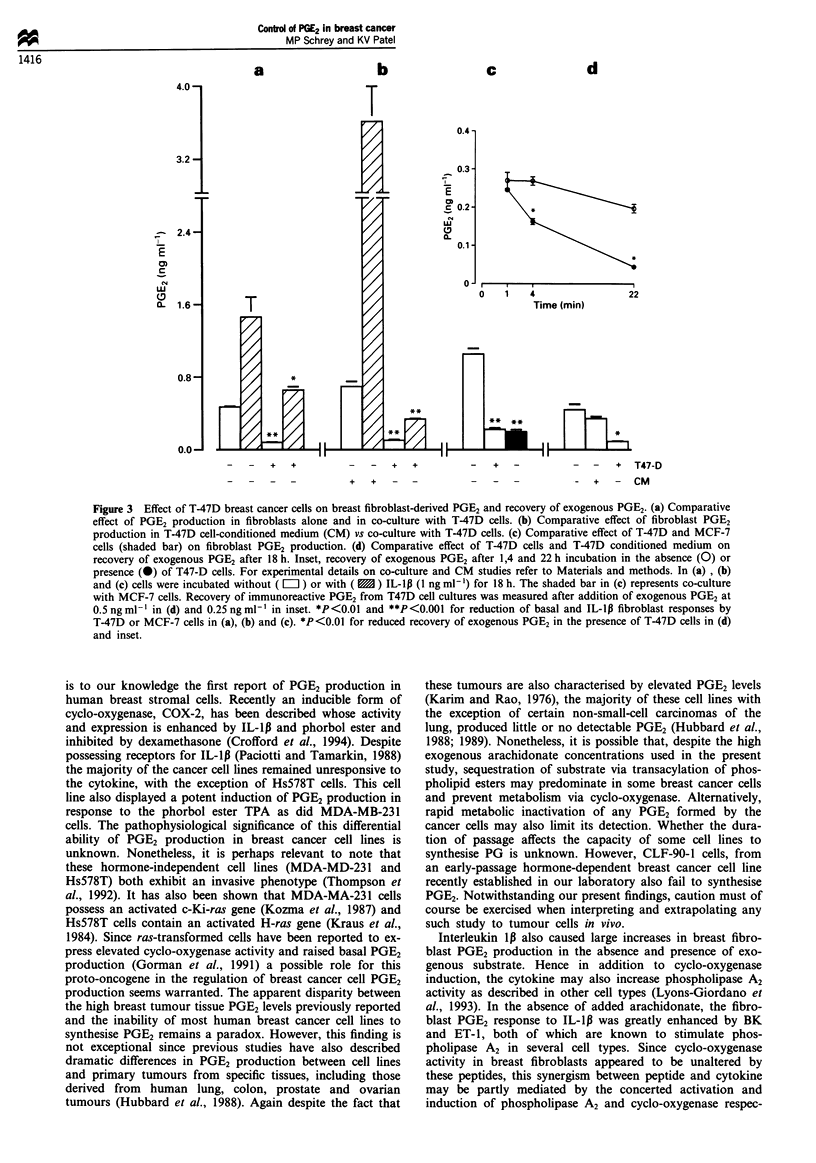
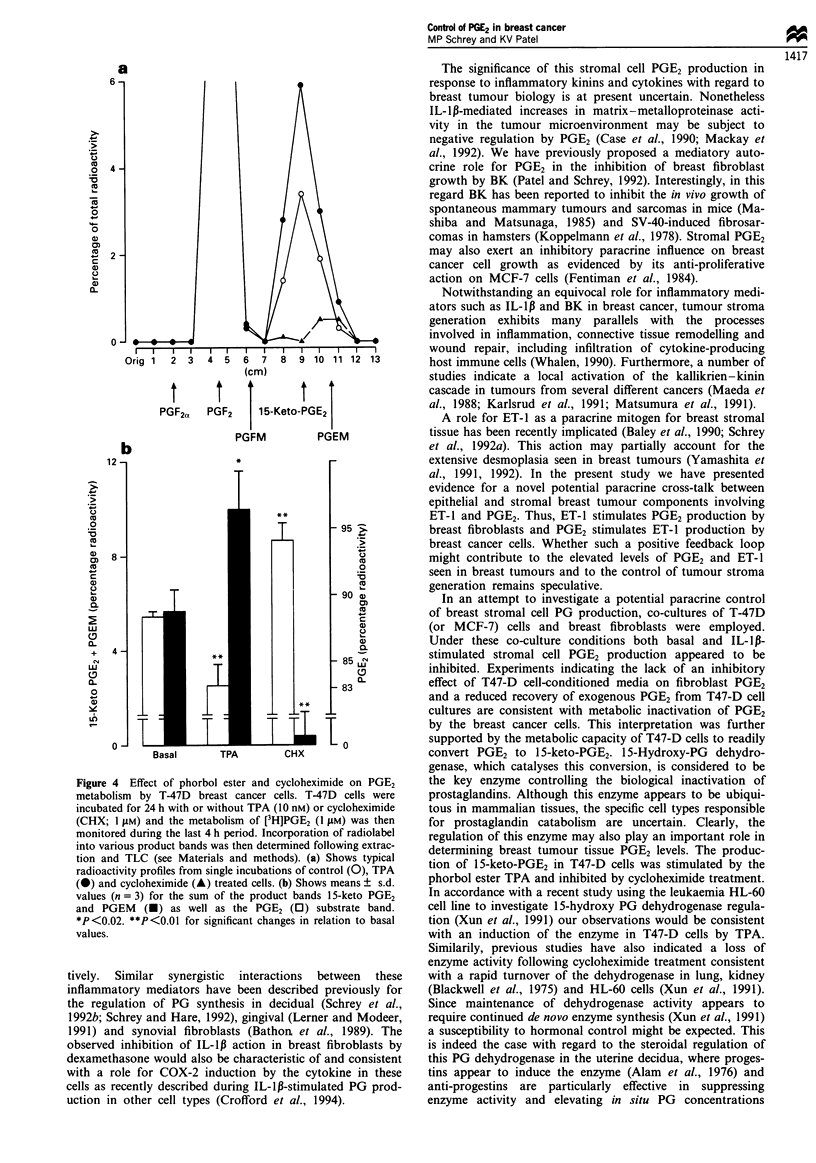
Malignant human breast tumours contain high levels of prostaglandin E2 (PGE2). However, the mechanisms controlling PGE2 production in breast cancer are unknown. This in vitro study investigates the capacity for PGE2 synthesis and metabolism in several human breast cancer cell lines and early passage human breast fibroblasts and seeks to identify potential regulatory factors which may control these pathways. Basal PGE2 production rose up to 30-fold in breast fibroblast lines on addition of exogenous arachidonic acid (10 microM), whereas no such changes were observed in six out of seven cancer cell lines, with the exception of modest increases in MDA-MB-231 cells. Interleukin 1 beta (IL-1 beta) also induced PGE2 production in breast fibroblasts in the presence of excess substrate, consistent with cyclo-oxygenase induction by the cytokine. Under these conditions only Hs578T cells and MDA-MB-231 cells demonstrated large increases in PGE2 in response to IL-1 beta or phorbol ester; no such responses were seen in MCF-7, T47-D, ZR-75-1, BT-20 or CLF-90-1 cells. In the absence of added arachidonate, bradykinin (BK) and endothelin-1 (ET-1), potentiated PGE2 production in IL-1 beta-treated fibroblasts, possibly by mobilising endogenous substrate. PGE2 also stimulated ET-1 production by breast cancer cells. In co-cultures with T47-D cells both basal and stimulated PGE2 production by breast fibroblasts was greatly reduced. This appeared to be due to metabolic inactivation by the cancer cell since T47-D cells readily converted PGE2 to 15-keto-PGE2. This apparent 15-hydroxy-PG dehydrogenase activity was stimulated by TPA and inhibited by cycloheximide. In conclusion, breast fibroblasts, particularly under the influence of inflammatory mediators, provide a potentially rich source for PGE2 production in breast tumours, whereas significant contributions from the epithelial tumour component may be restricted to cancer cells exhibiting an invasive phenotype. Metabolic inactivation by the cancer cells may also play an important role in the regulation of breast tumour PGE2 levels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam N. A., Russell P. T., Tabor M. W., Moulton B. C. Progesterone and estrogen control of uterine prostaglandin dehydrogenase activity during deciduomal growth. Endocrinology. 1976 Apr;98(4):859–863. doi: 10.1210/endo-98-4-859. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A., Cramer S., Bandyopadhyay G. K., Imagawa W., Yang J., Elias J., Beattie C. W., Das Gupta T. K., Nandi S. Differential proliferative response to linoleate in cultures of epithelial cells from normal human breast and fibroadenomas. Cancer Res. 1989 Feb 15;49(4):857–862. [PubMed] [Google Scholar]
- Baley P. A., Resink T. J., Eppenberger U., Hahn A. W. Endothelin messenger RNA and receptors are differentially expressed in cultured human breast epithelial and stromal cells. J Clin Invest. 1990 Apr;85(4):1320–1323. doi: 10.1172/JCI114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay G. K., Imagawa W., Wallace D., Nandi S. Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem. 1987 Feb 25;262(6):2750–2756. [PubMed] [Google Scholar]
- Bathon J. M., Proud D., Krackow K., Wigley F. M. Preincubation of human synovial cells with IL-1 modulates prostaglandin E2 release in response to bradykinin. J Immunol. 1989 Jul 15;143(2):579–586. [PubMed] [Google Scholar]
- Bennett A., Charlier E. M., McDonald A. M., Simpson J. S., Stamford I. F., Zebro T. Prostaglandins and breast cancer. Lancet. 1977 Sep 24;2(8039):624–626. doi: 10.1016/s0140-6736(77)92496-5. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J., Vane J. R. Rapid reduction of prostaglandin 15-hydroxy dehydrogenase activity in rat tissues after treatment with protein synthesis inhibitors. Br J Pharmacol. 1975 Oct;55(2):233–238. doi: 10.1111/j.1476-5381.1975.tb07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R. Elevated cAMP is required for stimulation of eicosanoid synthesis by interleukin 1 and bradykinin in BALB/c 3T3 fibroblasts. J Cell Physiol. 1992 Jun;151(3):512–518. doi: 10.1002/jcp.1041510310. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Kumkumian G. K., Remmers E. F., Wilder R. L. IL-1 regulation of transin/stromelysin transcription in rheumatoid synovial fibroblasts appears to involve two antagonistic transduction pathways, an inhibitory, prostaglandin-dependent pathway mediated by cAMP, and a stimulatory, protein kinase C-dependent pathway. J Immunol. 1990 Dec 1;145(11):3755–3761. [PubMed] [Google Scholar]
- Cheng L., Kelly R. W., Thong K. J., Hume R., Baird D. T. The effects of mifepristone (RU486) on prostaglandin dehydrogenase in decidual and chorionic tissue in early pregnancy. Hum Reprod. 1993 May;8(5):705–709. doi: 10.1093/oxfordjournals.humrep.a138124. [DOI] [PubMed] [Google Scholar]
- Crofford L. J., Wilder R. L., Ristimäki A. P., Sano H., Remmers E. F., Epps H. R., Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994 Mar;93(3):1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentiman I. S., Duhig T., Griffiths A. B., Taylor-Papadimitriou J. Cyclic AMP inhibits the growth of human breast cancer cells in defined medium. Mol Biol Med. 1984 Apr;2(2):81–88. [PubMed] [Google Scholar]
- Foecking M. K., Kibbey W. E., Abou-Issa H., Matthews R. H., Minton J. P. Hormone dependence of 7,12-dimethylbenz[a]anthracene-induced mammary tumor growth: correlation with prostaglandin E2 content. J Natl Cancer Inst. 1982 Aug;69(2):443–446. [PubMed] [Google Scholar]
- Fulton A. M., Heppner G. H. Relationships of prostaglandin E and natural killer sensitivity to metastatic potential in murine mammary adenocarcinomas. Cancer Res. 1985 Oct;45(10):4779–4784. [PubMed] [Google Scholar]
- Fulton A. M., Zhang S. Z., Chong Y. C. Role of the prostaglandin E2 receptor in mammary tumor metastasis. Cancer Res. 1991 Apr 15;51(8):2047–2050. [PubMed] [Google Scholar]
- Gorman R. R., Bienkowski M. J., Lin A. H. Elevated prostaglandin H synthase gene expression in ras-transformed cells. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:73–76. [PubMed] [Google Scholar]
- Hubbard W. C., Alley M. C., Gray G. N., Green K. C., McLemore T. L., Boyd M. R. Evidence for prostanoid biosynthesis as a biochemical feature of certain subclasses of non-small cell carcinomas of the lung as determined in established cell lines derived from human lung tumors. Cancer Res. 1989 Feb 15;49(4):826–832. [PubMed] [Google Scholar]
- Hubbard W. C., Alley M. C., McLemore T. L., Boyd M. R. Profiles of prostaglandin biosynthesis in sixteen established cell lines derived from human lung, colon, prostate, and ovarian tumors. Cancer Res. 1988 Sep 1;48(17):4770–4775. [PubMed] [Google Scholar]
- Karlsrud T. S., Buø L., Aasen A. O., Johansen H. T. Characterization of kininogens in human malignant ascites. Thromb Res. 1991 Sep 15;63(6):641–650. doi: 10.1016/0049-3848(91)90090-j. [DOI] [PubMed] [Google Scholar]
- Karmali R. A., Welt S., Thaler H. T., Lefevre F. Prostaglandins in breast cancer: relationship to disease stage and hormone status. Br J Cancer. 1983 Nov;48(5):689–696. doi: 10.1038/bjc.1983.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. W., Deam S., Cameron M. J., Seamark R. F. Measurement by radioimmunoassay of prostaglandins as their methyl oximes. Prostaglandins Leukot Med. 1986 Sep;24(1):1–14. doi: 10.1016/0262-1746(86)90201-5. [DOI] [PubMed] [Google Scholar]
- Koppelmann L. E., Moore T. C., Porter D. D. Increased plasma kallikrein activity and tumour growth suppression associated with intralesional bradykinin injections in hamsters. J Pathol. 1978 Sep;126(1):1–10. doi: 10.1002/path.1711260102. [DOI] [PubMed] [Google Scholar]
- Kozma S. C., Bogaard M. E., Buser K., Saurer S. M., Bos J. L., Groner B., Hynes N. E. The human c-Kirsten ras gene is activated by a novel mutation in codon 13 in the breast carcinoma cell line MDA-MB231. Nucleic Acids Res. 1987 Aug 11;15(15):5963–5971. doi: 10.1093/nar/15.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerner U. H., Modéer T. Bradykinin B1 and B2 receptor agonists synergistically potentiate interleukin-1-induced prostaglandin biosynthesis in human gingival fibroblasts. Inflammation. 1991 Dec;15(6):427–436. doi: 10.1007/BF00923340. [DOI] [PubMed] [Google Scholar]
- Lyons-Giordano B., Pratta M. A., Galbraith W., Davis G. L., Arner E. C. Interleukin-1 differentially modulates chondrocyte expression of cyclooxygenase-2 and phospholipase A2. Exp Cell Res. 1993 May;206(1):58–62. doi: 10.1006/excr.1993.1120. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Ballin M., Pelina M. D., Farina A. R., Nason A. M., Hartzler J. L., Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. 1992;12(3-4):168–184. [PubMed] [Google Scholar]
- Maeda H., Matsumura Y., Kato H. Purification and identification of [hydroxyprolyl3]bradykinin in ascitic fluid from a patient with gastric cancer. J Biol Chem. 1988 Nov 5;263(31):16051–16054. [PubMed] [Google Scholar]
- Mashiba H., Matsunaga K. Tumor-inhibitory effect of intralesional injection of bradykinin and immunostimulants in mice. Cancer Lett. 1985 Nov;29(2):177–182. doi: 10.1016/0304-3835(85)90156-9. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Maruo K., Kimura M., Yamamoto T., Konno T., Maeda H. Kinin-generating cascade in advanced cancer patients and in vitro study. Jpn J Cancer Res. 1991 Jun;82(6):732–741. doi: 10.1111/j.1349-7006.1991.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B. F., Rogers K., Wong S. The dynamics of prostaglandin metabolism in human fetal membranes and decidua around the time of parturition. J Clin Endocrinol Metab. 1993 Sep;77(3):759–764. doi: 10.1210/jcem.77.3.8370697. [DOI] [PubMed] [Google Scholar]
- Paciotti G. F., Tamarkin L. Interleukin-1 directly regulates hormone-dependent human breast cancer cell proliferation in vitro. Mol Endocrinol. 1988 May;2(5):459–464. doi: 10.1210/mend-2-5-459. [DOI] [PubMed] [Google Scholar]
- Patel K. V., Schrey M. P. Human breast cancer cells contain a phosphoramidon-sensitive metalloproteinase which can process exogenous big endothelin-1 to endothelin-1: a proposed mitogen for human breast fibroblasts. Br J Cancer. 1995 Mar;71(3):442–447. doi: 10.1038/bjc.1995.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. V., Schrey M. P. Inhibition of DNA synthesis and growth in human breast stromal cells by bradykinin: evidence for independent roles of B1 and B2 receptors in the respective control of cell growth and phospholipid hydrolysis. Cancer Res. 1992 Jan 15;52(2):334–340. [PubMed] [Google Scholar]
- Raz A., Wyche A., Fu J., Seibert K., Needleman P. Regulation of prostanoids synthesis in human fibroblasts and human blood monocytes by interleukin-1, endotoxin, and glucocorticoids. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:22–27. [PubMed] [Google Scholar]
- Rolland P. H., Martin P. M., Jacquemier J., Rolland A. M., Toga M. Prostaglandin in human breast cancer: Evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. J Natl Cancer Inst. 1980 May;64(5):1061–1070. [PubMed] [Google Scholar]
- Schrey M. P., Hare A. Endothelin-1 stimulates phospholipid hydrolysis and prostaglandin F2 alpha production in primary human decidua cell cultures. Prostaglandins Leukot Essent Fatty Acids. 1992 Dec;47(4):321–325. doi: 10.1016/0952-3278(92)90205-w. [DOI] [PubMed] [Google Scholar]
- Schrey M. P., Holt J. R., Cornford P. A., Monaghan H., al-Ubaidi F. Human decidua is a target tissue for bradykinin and kallikrein: phosphoinositide hydrolysis accompanies arachidonic acid release in uterine decidua cells in vitro. J Clin Endocrinol Metab. 1992 Feb;74(2):426–435. doi: 10.1210/jcem.74.2.1309839. [DOI] [PubMed] [Google Scholar]
- Schrey M. P., Patel K. V., Tezapsidis N. Bombesin and glucocorticoids stimulate human breast cancer cells to produce endothelin, a paracrine mitogen for breast stromal cells. Cancer Res. 1992 Apr 1;52(7):1786–1790. [PubMed] [Google Scholar]
- Tan W. C., Privett O. S., Goldyne M. E. Studies of prostaglandins in rat mammary tumors induced by 7,12-dimethylbenz(a)anthracene. Cancer Res. 1974 Dec;34(12):3229–3231. [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Watson D. M., Kelly R. W., Miller W. R. Prostaglandins and prognosis in human breast cancer. Br J Cancer. 1987 Sep;56(3):367–370. doi: 10.1038/bjc.1987.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Chuah S. Y. Prostaglandins, steroids and human mammary cancer. Eur J Cancer Clin Oncol. 1985 Sep;21(9):1051–1055. doi: 10.1016/0277-5379(85)90290-1. [DOI] [PubMed] [Google Scholar]
- Watson J., Chuah S. Y. Technique for the primary culture of human breast cancer cells and measurement of their prostaglandin secretion. Clin Sci (Lond) 1992 Sep;83(3):347–352. doi: 10.1042/cs0830347. [DOI] [PubMed] [Google Scholar]
- Whalen G. F. Solid tumours and wounds: transformed cells misunderstood as injured tissue? Lancet. 1990 Dec 15;336(8729):1489–1492. doi: 10.1016/0140-6736(90)93188-u. [DOI] [PubMed] [Google Scholar]
- Xun C. Q., Tian Z. G., Tai H. H. Stimulation of synthesis de novo of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase in human promyelocytic leukaemia (HL-60) cells by phorbol ester. Biochem J. 1991 Oct 15;279(Pt 2):553–558. doi: 10.1042/bj2790553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Ogawa M., Egami H., Matsuo S., Kiyohara H., Inada K., Yamashita S., Fujita S. Abundant expression of immunoreactive endothelin 1 in mammary phyllodes tumor: possible paracrine role of endothelin 1 in the growth of stromal cells in phyllodes tumor. Cancer Res. 1992 Jul 15;52(14):4046–4049. [PubMed] [Google Scholar]
- Yamashita J., Ogawa M., Inada K., Yamashita S., Matsuo S., Takano S. A large amount of endothelin-1 is present in human breast cancer tissues. Res Commun Chem Pathol Pharmacol. 1991 Dec;74(3):363–369. [PubMed] [Google Scholar]
- Yamashita S., Yamashita J., Sakamoto K., Inada K., Nakashima Y., Murata K., Saishoji T., Nomura K., Ogawa M. Increased expression of membrane-associated phospholipase A2 shows malignant potential of human breast cancer cells. Cancer. 1993 May 15;71(10):3058–3064. doi: 10.1002/1097-0142(19930515)71:10<3058::aid-cncr2820711028>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]


