Abstract
Antibodies immobilized on the wall of a flow chamber can support leukocyte rolling in shear flow. IgM mAb to Lewisx (CD15) and sialyl Lewisx (CD15s), which are carbohydrate antigens related to selectin ligands, plus mAb to CD48 and CD59, could mediate rolling. IgM and IgG mAb to L-selectin (CD62L), lymphocyte function-associated antigen 1 (CD11a), CD43, intercellular adhesion molecule 3 (CD50), and CD45 mediated only firm adhesion. In contrast to selectins, antibodies supported rolling only within a restricted range of site densities and wall shear stresses, outside of which firm adhesion or detachment occurred. When wall shear stress was increased, rolling velocity increased rapidly for antibodies but not for selectins. The kinetics of dissociation from the substrate of transiently tethered cells also increased more rapidly as a function of shear stress for antibodies than for selectins. These comparisons emphasize a number of remarkable features of selectins, including the lack of development of firm adhesion, and suggest that specialized molecular or cellular mechanisms must be required to explain their ability to support rolling over a wide range of environmental variables.
In the first step in accumulation in inflammatory sites and homing to lymphoid tissues, circulating leukocytes tether to the vessel wall and then roll in response to hydrodynamic drag forces (1, 2). During rolling, the adhesive contact zone between the cell and the vessel is rapidly translated along the vessel wall. This requires the rapid breakage and formation of adhesive bonds and that the rate of bond formation keep up with the rate of bond breakage. Only certain adhesion molecules, including selectins, some integrins, and CD44 have been found to support rolling (1, 3–5). By contrast, many adhesion molecules, including integrins but not selectins, support another class of adhesion termed “firm adhesion,” which often involves cell spreading and cell migration.
Thus far, little is known about the characteristics that determine whether adhesion molecules support rolling adhesion or firm adhesion. It has been hypothesized that fast bond dissociation and association rates are important for rolling (6), and measurements on P-selectin are consistent with this idea (7, 8). However, the interaction of CD2 with lymphocyte function-associated 3 (9, 10) and binding of an IgE antibody to its antigen (11) have similar kinetics but do not appear to support rolling. Another factor that may be important is the effect of force on bond association and dissociation kinetics (12). The effect of force has been measured on the duration of transient tethers of cells to the vessel wall, which occurs at selectin densities below the minimum required to support rolling. The rate of dissociation of P-selectin tethers is increased only modestly by hydrodynamic force (8), which would contribute to the stability of rolling adhesions.
To allow comparisons to be made between molecules that are and are not physiologically specialized for rolling, we have tested whether antibodies can support leukocyte rolling. We have identified mAb, all of the IgM class, to carbohydrate antigens CD15 and CD15s and to the glycoproteins CD48 and CD59 that support tethering and rolling. However, rolling on antibodies is unstable and occurs only over a narrow range of substrate densities and wall shear stresses, and tethers have low mechanical stability. The results suggest that robust rolling behavior as exhibited by selectins requires molecular specializations.
MATERIALS AND METHODS
mAb were from the 5th International Leukocyte Workshop. Cell lines were maintained in RPMI 1640 medium/10% fetal bovine serum. Jurkat mock (JPuro) and fucosyl–transferase V (JFT5) transfectants were prepared as described (13) and were maintained in the same medium with 10 μg/ml puromycin. Neutrophil preparations (6) and immunofluorescent flow cytometry (13) were as described. A polystyrene Petri dish was coated with a ≈5-mm diameter, 20-μl spot of purified mAb (5 μg·ml−1 unless specified otherwise) or 0.75 μg·ml−1 of E-selectin (14) in PBS (pH 9) for 1 h at 37°C, followed with 2% BSA/PBS (pH 7.4) for 1 h at 37°C to block nonspecific binding sites, assembled in a parallel plate flow chamber, and mounted on the stage of an inverted phase contrast microscope (6). Cell lines were resuspended in Hank’s balanced salt solution/10 mM Hepes (pH 7.4) and perfused into the flow chamber in Hank’s balanced salt solution/Hepes with 5 mM EDTA for adhesion to mAb or 2 mM Ca2+ for adhesion to E-selectin at a wall shear stress of 0.25 dyn·cm−2 for 30 s. Then the shear stress was increased in steps every 10 s. Microscopic images of cells were videotaped for later analysis. mAb site density was determined essentially as described for E-selectin (14) as the number of 125I-labeled rat anti-murine κ light chain mAb 187.1 (15) (22 μCi/μg) molecules bound per square micrometer. Test antibodies were coated on individual Immulon 3 plastic microtiter wells (Immulon, Chantilly, VA) at varying concentrations and blocked with 1% heat-treated human serum albumin in PBS. After washing with human serum albumin in PBS, 40 μl of 125I-187.1 diluted to 7.5 μg/ml in human serum albumin/PBS (pH 7.4) was added for 1 h at 4°C. Wells were washed, separated, and counted. Nonspecific binding in the absence of first antibody was subtracted.
A computerized imaging system consisting of a Pentium computer with modular vision computer boards (150/40-VL subsystem; Imaging Technology, Bedford, MA) and software developed by us for analysis of rolling adhesion and transient tethering in real time will be described elsewhere. Images of cells were segmented from the background, and motions of individual cells were analyzed between each 0.033-s video frame. Cells interacting with the substrate were detected by discriminating their velocity from the hydrodynamic velocity distribution of cells free in flow. Adherent cells were tracked to generate a trajectory map for 8 s.
For transient tethers (8), PM-81 mAb was diluted to 0.3, 0.2, 0.1, and 0.06 μg·ml−1 in PBS supplemented with 10 μg·ml−1 BSA to prevent nonuniform distribution on the substrate, which occurred at antibody concentrations below 5 μg·ml−1. Transient tethering events (≈100–3000) were recognized for each experimental group, and the lifetime of each tether was measured. The most rapidly dissociating 90% of arrested cells was used for determining koff. The resolution in measurements of the duration of transient tethers was dependent on the flow velocity of cells and was ±0.13 s at 0.1 dyn·cm−2, ±0.066 s at 0.2 dyn·cm−2, and ±0.033 s (one video frame) at ≥0.3 dyn·cm−2.
The hydrodynamic force (Fs) on a tethered cell and the force on the tether bond were calculated as described (8). The lever arm l, i.e., the distance between the tether point and the projection of the center of the cell on the substrate, was determined by measuring the distance of 2l traveled by transiently tethered cells when flow was reversed, as described elsewhere (R.A., S.C., K. D. Puri, E. B. Finger, and T.A.S., unpublished work).
RESULTS
To identify mAb that could mediate rolling, we first tested mAb to surface structures involved in adhesive interactions. Selectins bind to carbohydrate ligands in which the sialyl Lewisx (CD15s) moiety is of key importance. Of interest, the CSLEX-1 mAb to sialyl Lewisx [and, with varying efficiency, four mAb to Lewisx (CD15)] supported rolling adhesions (Table 1). One mAb to Lewisa,b supported slow rolling. By contrast, mAb to CD62L (L-selectin) [as reported (16)], the IgS super family adhesion molecule CD50, the integrin CD11a, the mucin CD43, and surface glycoproteins CD45, CD55, and CD108 could mediate firm adhesion but not rolling. CD15 and CD15s are recognized by IgM mAb and are abundant on cell surfaces, so IgM mAbs to abundant surface glycoproteins were tested (Table 1). Of these, mAb to CD48 and CD59 were found to support rolling. Both molecules have glycosyl phosphatidylinositol membrane anchors, but whether this has significance for rolling is unclear. Despite the ability of L-selectin to mediate rolling on carbohydrate ligands (17, 18), mAbs DREG56 and DREG200 to L-selectin did not support rolling even when tested over a range of coating densities (not shown), implying that the ability to mediate rolling does not correlate to the structure or location of the cell surface antigen/receptor alone (16).
Table 1.
Adhesive interactions of cells with immobilized antibodies
| Antigen | Antibody
|
Antigen expression
|
Adhesion† | ||
|---|---|---|---|---|---|
| Name | Class | Cell line | MESF* | ||
| CD15s | CSLEX-1 | IgM | JFT5 | 1188 | R |
| HL-60 | 6433 | R | |||
| JPuro | 0 | N | |||
| CD15 | PM-81 | IgM | PMN | 2133 | R |
| JFT5 | 3951 | R | |||
| HL-60 | 7079 | R | |||
| JPuro | 270 | R | |||
| CD15 | IGR-15B6 | IgM | JFT5 | 2530 | R |
| HL-60 | 8828 | R | |||
| JPuro | 343 | N | |||
| CD15 | mAb80 | IgM | JFT5 | 1649 | T/R |
| HL-60 | 6187 | T/R | |||
| JPuro | 29 | N | |||
| CD15 | 2TAP146 | IgM | JFT5 | 459 | T/R |
| HL-60 | 7350 | T/R | |||
| JPuro | 2 | N | |||
| Lewisa,b | HEA164 | IgM | RD3/5 | 2380 | r |
| Hela | 7 | N | |||
| CD108 | MEM-121 | IgM | JY | 139 | F/r |
| CD48 | MEM-124 | IgM | JY | 2514 | R |
| CD59 | MEM-125 | IgM | Hela | 7495 | r |
| HL-60 | 554 | R | |||
| CD59 | C2G4 | IgM | Hela | 6526 | F/r |
| HL-60 | 620 | F/r | |||
| CD55 | MEM-118 | IgM | JY | 293 | F |
| HL-60 | 109 | F | |||
| JPuro | 172 | F | |||
| CD45 | NK45 | IgM | HL-60 | 881 | F |
| JPuro | 1678 | F | |||
| CD50 | CBR-IC3/1 | IgG | HL-60 | 663 | F |
| JPuro | 581 | F | |||
| CD50 | CBR-IC3/2 | IgG | HL-60 | 652 | F |
| JPuro | 644 | F | |||
| CD50 | CBR-IC3/6 | IgM | HL-60 | 391 | F |
| JPuro | 362 | F | |||
| CD11a | CBR LFA-1/10 | IgM | KG1a | 777 | F |
| HL-60 | 369 | F | |||
| CD11a | CBR LFA-1/9 | IgM | KG1a | 1475 | F |
| HL-60 | 554 | F/r | |||
| JPuro | 128 | F/r | |||
| CD11a | CBR LFA-1/1 | IgM | KG1a | 1150 | F |
| HL-60 | 458 | F | |||
| JPuro | 117 | F | |||
| CD62L | DREG56 | IgG | JPuro | 309 | F |
| HL-60 | 18 | N | |||
| CD62L | DREG200 | IgG | JPuro | 142 | F |
| CD62L | LAM1-3 | IgG | JPuro | 419 | F/r |
| CD43 | L10 | IgG | KG1a | 1056 | F |
| HL-60 | 1944 | F | |||
| JPuro | 582 | F | |||
| CD43 | BRA7G | IgM | KG1a | 1144 | F |
Mean immunofluorescence intensity was standardized with fluorescein isothiocyanate-conjugated beads from Flow Cytometry Standards (San Juan, Puerto Rico) to calculate molecules of equivalent soluble fluorochrome ×10−3 (MESF).
Interactions were classified based on the motion of cells tethered to the substrate in shear flow and subjected to subsequent increases in shear. R, rolling, at least 50% of attached cells would eventually roll for more than five cell diameters with a velocity greater than 1 μm s−1 before detachment; r, slow rolling, at least 50% of cells would roll for more than five cell diameters with a velocity less than 1 μm sec−1 before detachment; T, transient tethering, cells would be tethered to the substrate for a certain time and then detached in the flow, and the detached cells might be tethered again downstream; F, firm adhesion, attached cells stuck to the substrate until detachment by a higher shear; the cells moved less than two cell diameters before reaching the hydrodynamic velocity, and once detached the cells were rarely attached again; F/r, cells would slowly roll for two to five cell diameters at high shear before detachment; N, no adhesion, cells did not attach to the substrate.
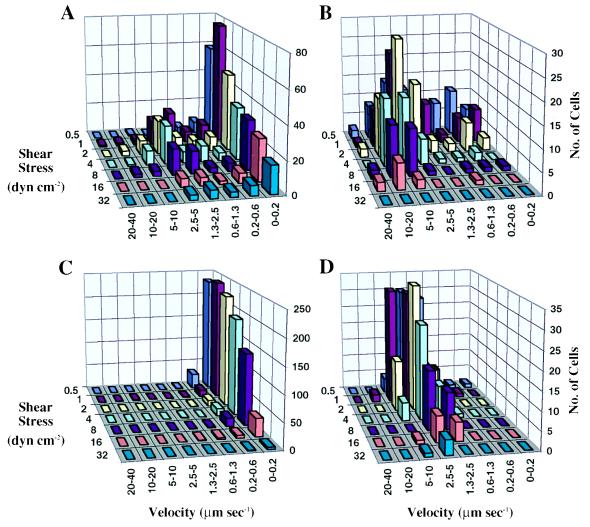
On selectins, all cells roll, and there is a uniphasic distribution of rolling velocities, as illustrated here with E-selectin (Figs. 1D and 2D). By contrast, cells on antibody substrates displayed a biphasic distribution of rolling velocities, with one phase corresponding to firmly adherent cells and another phase corresponding to rolling cells (Figs. 1 A and B and 2 A and B). The percentage of firmly adherent (velocity ≤ 0.6 μm·s−1) and rolling cells varied strongly with shear stress for antibodies (Fig. 2 A and B) but not for E-selectin (Fig. 2D). After tethering on mAb that could support rolling, cells often rolled with a jerky motion and then either accelerated and detached quickly or rolled a short distance and then stopped. As the shear stress was increased, the firmly adherent cells reinitiated rolling, thus increasing the fraction of rolling cells. Further increase in shear resulted in detachment of the rolling cells (Fig. 2 A and B).
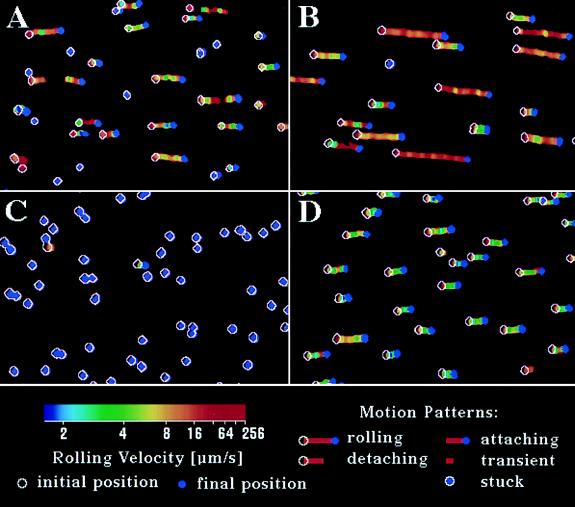
Figure 1.
Representative cell trajectory maps at a wall shear stress 4.4 dyn·cm−2 for 8 s. An open white circle and a solid purple spot indicate the initial and final positions of a cell, respectively, and a colored line shows the path of cells during the tracking period. Color is keyed to velocity. (A) HL-60 cells on CD15 mAb PM-81 (150 sites μm−2). (B) JY cells on CD48 mAb MEM-124 (180 sites μm−2). (C) Untransfected Jurkat cells on DREG56 mAb to L-selectin (150 sites μm−2). (D) HL-60 cells on E-selectin (399 sites μm−2).
Figure 2.
Rolling velocity distribution as a function of wall shear stress. The rolling velocity of individual cells was calculated from trajectory maps, and the number of cells within a given velocity range was counted to derive the population distribution. (A) HL-60 cells on CD15 mAb PM-81. (B) JY cells on CD48 mAb MEM-124. (C) Jurkat cells on DREG56 mAb to L-selectin. (D) HL-60 cells on E-selectin.
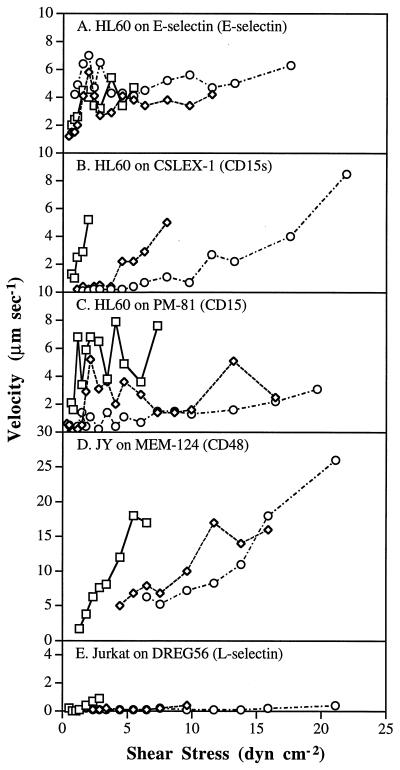
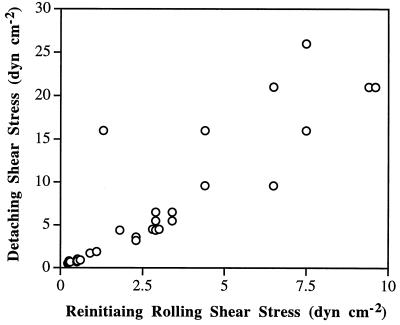
Rolling velocity increased more rapidly with increasing shear stress for antibody-mediated rolling than for E-selectin-mediated rolling. As previously reported for neutrophils rolling on selectins (6, 14, 18), the rolling velocity of HL-60 cells on E-selectin increased proportionally to shear stress at low shear levels and reached a near-plateau at high shear levels, as shown for the population as a whole (Fig. 2D) and for representative cells (Fig. 3A). Cells rolled as a group over the substrate with similar rolling velocities (Fig. 1D). In contrast, cells rolled on antibodies with wide variation in velocity, and some cells were stuck (Fig. 1 A and B). Observations on a large number of individual cells rolling on antibodies, a few of which are represented (Fig. 3 B–D), showed that rolling was initiated at different shear levels and velocity increased rapidly with increasing shear stress until the cells detached. The lower the shear stress required to initiate rolling, the faster the rolling velocity as a function of wall shear stress (Fig. 3 B–D). The shear at which rolling initiated correlated with the shear at which detachment occurred (Fig. 4). Thus, the range of shear within which individual cells rolled on antibodies was more restricted than on E-selectin.
Figure 3.
Individual cell rolling velocities as a function of shear stress. Three representative cells that detached at low, medial, or high shear stresses, respectively, were selected, and the rolling velocity was determined from the trajectory maps. The three types of symbols in each panel represent three individual cells, and detachment occurred when shear stress was incremented beyond the last point shown.
Figure 4.
The relationship between the shear stress at which cells reinitiated rolling and the resistance to detachment for HL-60 cells on mAb CSLEX-1. Cells were accumulated at 0.25 dyn/cm2 and became stuck. Shear was subsequently incrementally increased; points represent, for individual cells, the shear stress at which rolling was reinitiated and detachment occurred.
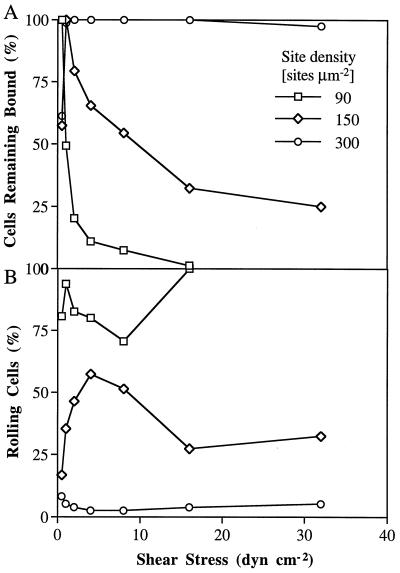
A site density-dependent phase transition between rolling and firm adhesion was seen on antibodies, which never has been seen on selectins and their ligands. Transient tethering at low site densities was gradually converted to rolling on PM-81 mAb when the density on the substrate was increased over 50 IgM κ light chain sites μm−2. The most favorable density for rolling on PM-81 mAb was at ≈100–150 sites μm−2, and a modest further increase to 300 sites/μm2 resulted in a dramatic increase in resistance to detachment by shear (Fig. 5A) and a sharp decline in the percentage of rolling cells (Fig. 5B). A similar transition from rolling to firm adhesion at higher coating densities was seen for other antibodies tested, including CSLEX-1 (data not shown). In contrast, neutrophils could roll on E-selectin over a range of 35–900 sites μm−2 (14).
Figure 5.
The effect of site density on rolling of HL-60 cells on CD15 mAb PM-81. (A) Percentage of cells that remain adherent as shear stress is increased. (B) Percentage of adherent cells that roll.
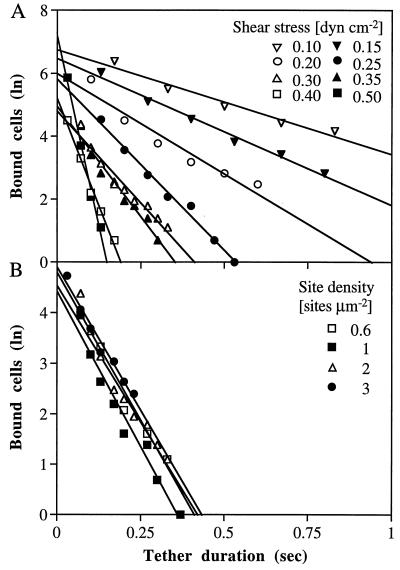
To test whether antibody–antigen tether bonds have a lower resistance to tensile force than selectin tether bonds, we studied the effect of tensile force on the duration of transient tethers, i.e., pauses of arrest on the substrate separated by movement at the hydrodynamic velocity that occur at low ligand site densities. Not all of the antibodies that mediated rolling supported transient tethering at low substrate site densities. HL-60 and Jurkat fucosyl–transferase V transfectant JFT5 cells transiently tethered well on CD15 mAb PM-81 but poorly on CD15s mAb CSLEX-1. CD48 mAb MEM-124 also supported transient tethering of JY cells. No transient tethering events were observed on antibodies that supported only firm adhesions at higher site densities. The duration of HL-60 cell tethering events was measured at various wall shear stresses on CD15 mAb PM-81 densities ranging from 0.6 to 3 sites μm−2, i.e., below the density required for rolling (Fig. 6 A and B). Greater than 90% of the cell arrests in each data set demonstrated first-order dissociation kinetics. Furthermore, the cellular off rate, koff, i.e., the negative slope in kinetic plots, was identical for different site densities (Fig. 6B). This suggests that a quantal tethering unit accounts for approximately ≥90% of the arrests under these conditions. The remaining ≤10% of tether events dissociated more slowly and may represent cells that formed more than one tether.
Figure 6.
Kinetics of dissociation of transiently tethered HL-60 cells from CD15 mAb PM-81. (A) Tether dissociation as a function of shear stress on immobilized PM-81 mAb at a density of 2 κ light chain sites μm−2. (B) Tether dissociation as a function of site density at 0.3 dyn·cm−2. The natural logarithm of the number of cells that remain tethered is plotted as a function of time after initiation of the tether.
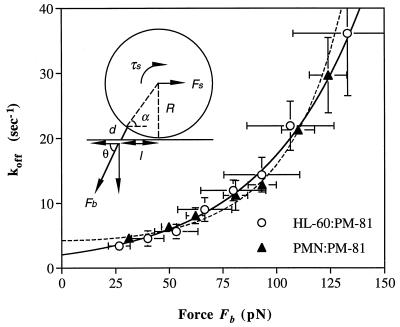
To determine the effect of the tensile force on koff, it was necessary to estimate the force on the tether bond, Fb. The hydrodynamic force on the cell, Fs, is proportional to wall shear stress and was estimated from Goldman’s equation (19). The relation between Fb and Fs is a function of the angle between them and hence of the lever arm, l (Fig. 7). The lever arm was determined by measuring the distance that transiently tethered cells moved during flow reversal. The lever arms of neutrophils and HL-60 cells on PM-81 antibody were 3.0 ± 0.38 μm and 4.7 ± 1.0 μm, respectively, with cell diameters of 8.5 ± 0.47 μm and 12.6 ± 1.6 μm, respectively. Because of their larger size, the hydrodynamic force on HL-60 cells and hence on the tether bond was ≈2-fold greater than on neutrophils at a given shear. Nonetheless, when wall shear stress was converted to Fb, the curves relating Fb and koff were superimposable (Fig. 7). The dissociation rate koff of PM-81 increased from ≈3 to 36 s−1 with an increase in the force on the bond, Fb, from 25 to 130 pN (Fig. 7). To characterize quantitatively the effect of force on koff, the data were fit to Bell’s model of koff as an exponential function of Fb (20) and a Hookean spring model of koff as an exponential function of Fb2 as proposed by Dembo et al. (21). χ2 analysis showed that the fits were good for the Bell model (χ2 = 56.2, with 112 df) and the Hookean spring model (χ2 = 82.4, with 112 df) for the combined data from HL-60 cells and neotrophils. The fit to the Bell model yields a dissociation rate in the absence of applied force, koff°, of 2.05 ± 0.12 s−1, and a bond interaction range σ, which represents the length scale over which receptor–ligand interaction occurs, of 0.88 ± 0.03 Å. The fit to the Hookean spring model yields koff° of 4.24 ± 0.29 s−1 and the value of the bond spring constant divided by the fractional spring slippage (21) κ/fκ of 0.92 ± 0.06 Nm−1. The resistance of koff to force may be termed “mechanical stability”; the inverse, the susceptibility to force, may be termed “reactive compliance.” The bond interaction range σ is a quantitative representation of the reactive compliance. The value of this constant shows that the CD15 mAb PM-81 tether bond is 4-fold more compliant than the L-selectin tether bond (σ = 0.19 Å) and 2-fold more compliant than the P-selectin (σ = 0.39 Å) and E-selectin (σ = 0.30 Å) tether bonds (R.A., S.C., K. D. Puri, E. B. Finger, and T.A.S., unpublished work and ref. 8).
Figure 7.
Dissociation rate as a function of the force on the tether bond. The koff was measured at different shear stresses for neutrophils and HL-60 cells tethered to PM-81 at different site densities. The force and torque balances on a tethered cell in shear flow are: Fb cosθ = Fs and Fb l sinθ = τs + R Fs. Using the data of cell diameter and the lever arm, the Goldman equation for calculation of Fs yielded d = 1.0 ± 0.2 μm, θ = 62.8 ± 1.9°, and Fb/Fs = 2.18 ± 0.13 for neutrophils; and d = 1.6 ± 0.5 μm, θ = 61.5 ± 2.5°, and Fb/Fs = 2.09 ± 0.19 for HL-60 cells. The koff as a function of force on the bond was fit to the Bell model (solid line): koff = koff° exp(σFb/kT), where koff° is the unstressed koff, σ is the bond interaction range, k is Boltzmann’s constant, and T is the absolute temperature. The koff as a function of force on the bond also was fit to the spring model (21) (dashed line): koff = koff° exp (fκFb2/2κkT), where fκ is the fractional spring slippage (21) and κ is the bond spring constant. Vertical error bars show SD for different experiments and measurements at different site densities; horizontal error bars show the estimated uncertainty in the value of Fb at different shear stresses.
DISCUSSION
We have identified antibodies that can mediate leukocyte rolling. This extends the class of molecules known to support rolling. Furthermore, the contrasts between selectin and antibody-mediated rolling are revealing and suggest that specializations may be required for rolling to occur over a wide range of shear stresses and of ligand or selectin densities, without a phase transition to firm adhesion. In previous studies, antibodies to dinitrophenol, CD8, L-selectin, and IgG and interaction of IgE with the IgE receptor have been found to give firm adhesion, with no evidence of rolling adhesion (11, 16, 22, 23). This is in agreement with our finding that only a subset of mAb is capable of supporting rolling.
The translation along the vessel wall of the adhesive contact zone during rolling requires that bonds be rapidly broken and formed. The rate of bond breakage must be balanced dynamically by the rate of new bond formation for rolling adhesion to be maintained. Changes in conditions such as shear force or receptor or ligand density may alter the rate of bond breakage or bond formation. If a new state can be reached in which the rate of bond formation and breakage is balanced, cells will continue to roll. Loss of the dynamic balance will lead to either firm adhesion or detachment. For example, if the rate of bond formation exceeds the rate of bond breakage, the cell should roll more slowly, allowing more time for bond formation, leading to progressive slowing and finally firm adhesion. The ability of cells to maintain a balance between bond breakage and formation in a moving contact zone under different environmental conditions may be termed the “stability of rolling adhesion.” Stability in rolling velocity is observed in vivo over a wide range of wall shear stresses (24) and may be important to allow rolling cells to survey vessel walls for inflammatory signals relatively independently of local hemodynamic conditions.
Both kinetic and mechanical factors may be important in determining whether the dynamic balance can be maintained, i.e., in determining the dynamic range over which rolling adhesion can occur. The PM-81 tether bond had a compliance that was low enough to enable transient tethers and rolling; it was nonetheless 4-fold more compliant than the L-selectin tether and 2-fold more compliant than the P-selectin and E-selectin tethers (R.A., S.C., K. D. Puri, E. B. Finger, and T.A.S., unpublished work and ref. 8). A higher reactive compliance will reduce the rolling stability by narrowing the range of shear stress for rolling adhesion. In other words, the lower σ of selectins than PM-81 mAb favors rolling over a wider range of wall shear stresses. This finding is consistent with the idea that the mechanical stability of selectins is important for their function in shear flow; however, measurements with a wider range of antibodies would be required to generalize the lower reactive compliance of selectins than antibodies and confirm this conclusion.
Mechanical or kinetic factors may explain the lack of detection of transient tethers with mAb that could only support firm adhesion. The bond life times of mAb with high reactive compliance would be greatly shortened in shear flow and might not be detectable on a video timescale of 0.033 s. Therefore, interactions through these mAb might not be detectable unless they were multivalent and gave rise to firm adhesion. Alternatively, fast kon and slow koff might favor firm adhesion once a transient tether was formed. In either case, it would be difficult to reach a state of dynamic balance.
The development of firm adhesion by neutrophils or HL-60 cells on CD15 mAb PM-81 substrates, but not on selectins, cannot be explained by differences in koff°. The koff° of mAb PM-81 of 2 s−1 is 2- to 3-fold faster than the koff° for P-selectin (8) and E-selectin (R.A., S.C., K. D. Puri, E. B. Finger, and T.A.S., unpublished work and ref. 25). If all other factors were equal, this faster koff should favor faster rolling not firm adhesion.
Other specializations may be required to explain the full range of differences between selectins and antibodies. Rolling on antibodies was not only limited to a narrow range of wall shear stresses but also to a narrow range of antibody densities on the substrate. A 3-fold difference in PM-81 antibody density led to almost complete transition between rolling and firm phases of adhesion. Rolling and firmly adherent populations of cells often coexisted. These are the types of results that are predicted based on dynamic simulations of cell adhesive interactions in shear flow (12) or by some peeling models (21) and contrast with results with selectins. One remarkable property of selectins is the lack of development of firm adhesion in the absence of shear flow. When flow is initiated, cells begin to roll on selectins immediately. The current studies on antibodies further emphasize the unusualness of this lack of adhesion strengthening. Adhesion strengthening was evident with antibodies after tethering at low shear when cells rolled a short distance and then stopped and by the requirement for shear to be raised above a threshold level for firmly adherent cells to resume rolling. Another remarkable feature is the near-plateau in rolling velocity seen with selectins. One factor that has been proposed to stabilize rolling velocity in vivo is deformation of cell shape with elongation in the direction of flow, resulting in a greater area of contact with the vessel wall (26). This may well be important but should apply equally well to rolling through selectins and antibodies and therefore is unlikely to explain the differences seen here.
It is interesting that, although the compliance and tether koff° of PM-81 mAb differ from E-selectin only by ≈2-fold, rolling on E-selectin is dramatically more stable. Thus, although we have found differences between antibodies and selectins that may contribute to differing dynamic behaviors, our comparisons also suggest that it may be important to search for other mechanisms that contribute to the stability of rolling on selectins.
Acknowledgments
We thank Dr. Chafen Lu for measurements of E-selectin site density. This work was supported by National Institutes of Health Grant HL-48675.
References
- 1.Springer T A. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 2.Lasky L A, Rosen S D. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J I, Goldsten I J, Snyderman R, editors. New York: Raven; 1992. pp. 407–439. [Google Scholar]
- 3.Tözeren A, Kleinman H K, Wu S, Mercurio A M, Byers S W. J Cell Sci. 1994;107:3153–3163. doi: 10.1242/jcs.107.11.3153. [DOI] [PubMed] [Google Scholar]
- 4.Degrendele H C, Estess P, Picker L J, Siegelman M H. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark R A, Alon R, Springer T A. J Cell Biol. 1996;134:1075–1087. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence M B, Springer T A. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 7.Ushiyama S, Laue T M, Moore K L, Erickson H P, McEver R P. J Biol Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
- 8.Alon R, Hammer D A, Springer T A. Nature (London) 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 9.Chan P-Y, Lawrence M B, Dustin M L, Ferguson L M, Golan D E, Springer T A. J Cell Biol. 1991;115:245–255. doi: 10.1083/jcb.115.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Merwe P A, Barclay A N, Mason D W, Davies E A, Morgan B P, Tone M, Krishnam A K C, Ianelli C, Davis S J. Biochemistry. 1994;33:10149–10160. doi: 10.1021/bi00199a043. [DOI] [PubMed] [Google Scholar]
- 11.Tempelman L A, Hammer D A. Biophys J. 1994;66:1231–1243. doi: 10.1016/S0006-3495(94)80907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer D A, Apte S M. Biophys J. 1992;63:35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuhlbrigge R C, Alon R, Puri K D, Lowe J B, Springer T A. J Cell Biol. 1996;135:837–848. doi: 10.1083/jcb.135.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence M B, Springer T A. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 15.Ware C F, Reade J L, Der L C. J Immunol Methods. 1984;74:93–104. doi: 10.1016/0022-1759(84)90371-5. [DOI] [PubMed] [Google Scholar]
- 16.von Andrian U H, Hasslen S R, Nelson R D, Erlandsen S L, Butcher E C. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 17.Berg E L, McEvoy L M, Berlin C, Bargatze R F, Butcher E C. Nature (London) 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence M B, Berg E L, Butcher E C, Springer T A. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 19.Goldman A J, Cox R G, Brenner H. Chem Eng Sci. 1967;22:653–660. [Google Scholar]
- 20.Bell G I. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 21.Dembo M, Torney D C, Saxman K, Hammer D. Proc R Soc London Ser B. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- 22.Pierres A, Tissot O, Malissen B, Bongrand P. J Cell Biol. 1994;125:945–953. doi: 10.1083/jcb.125.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierres A, Benoliel A-M, Bongrand P. J Biol Chem. 1995;270:26586–26592. doi: 10.1074/jbc.270.44.26586. [DOI] [PubMed] [Google Scholar]
- 24.Atherton A, Born G V R. J Physiol. 1973;233:157–165. doi: 10.1113/jphysiol.1973.sp010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplanski G, Farnarier C, Tissot O, Pierres A, Benoliel A-M, Alessi M-C, Kaplanski S, Bongrand P. Biophys J. 1993;64:1922–1933. doi: 10.1016/S0006-3495(93)81563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firrell J C, Lipowsky H H. Am J Physiol. 1989;256:H1667–H1674. doi: 10.1152/ajpheart.1989.256.6.H1667. [DOI] [PubMed] [Google Scholar]