Abstract
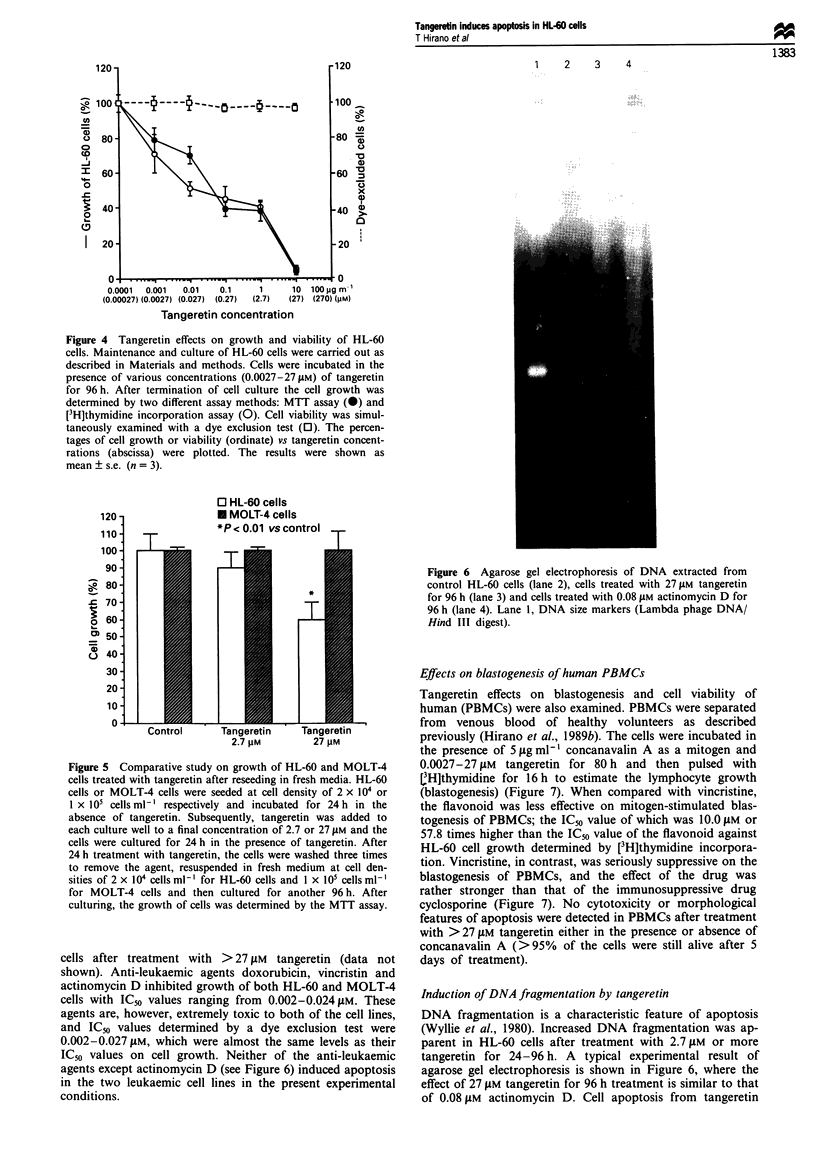
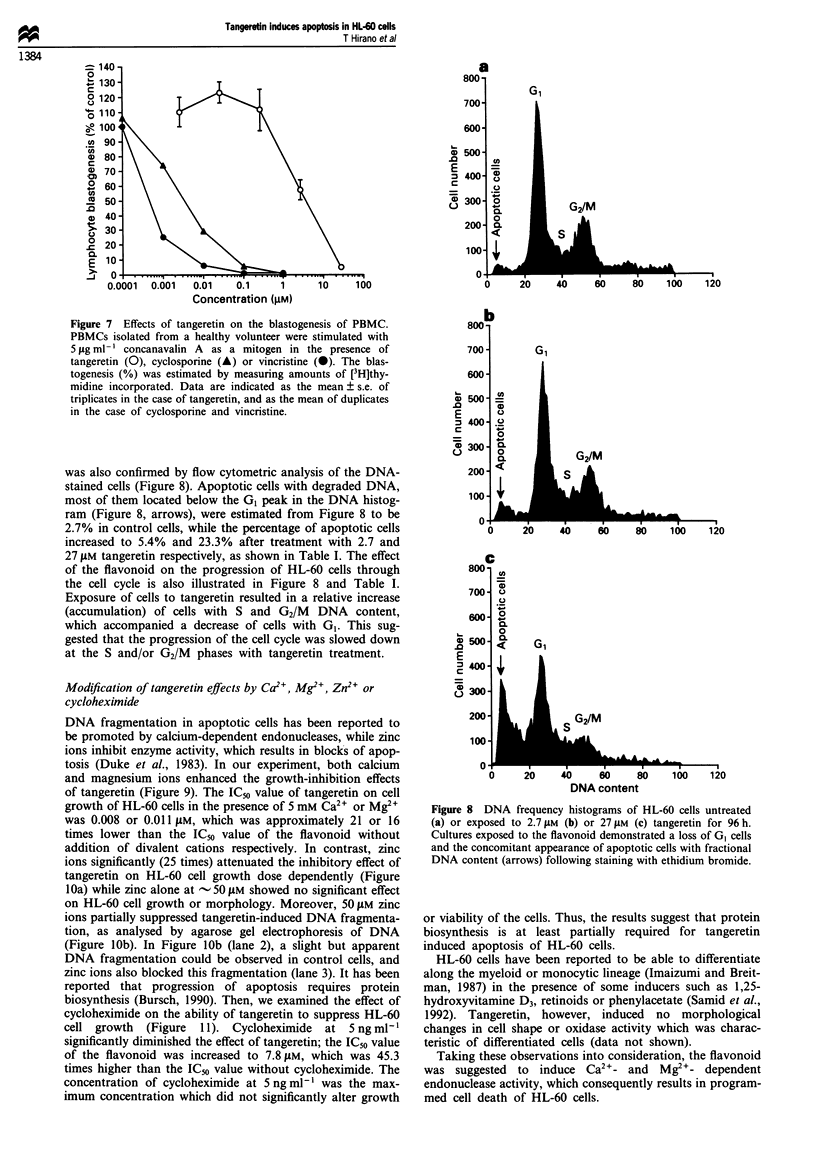
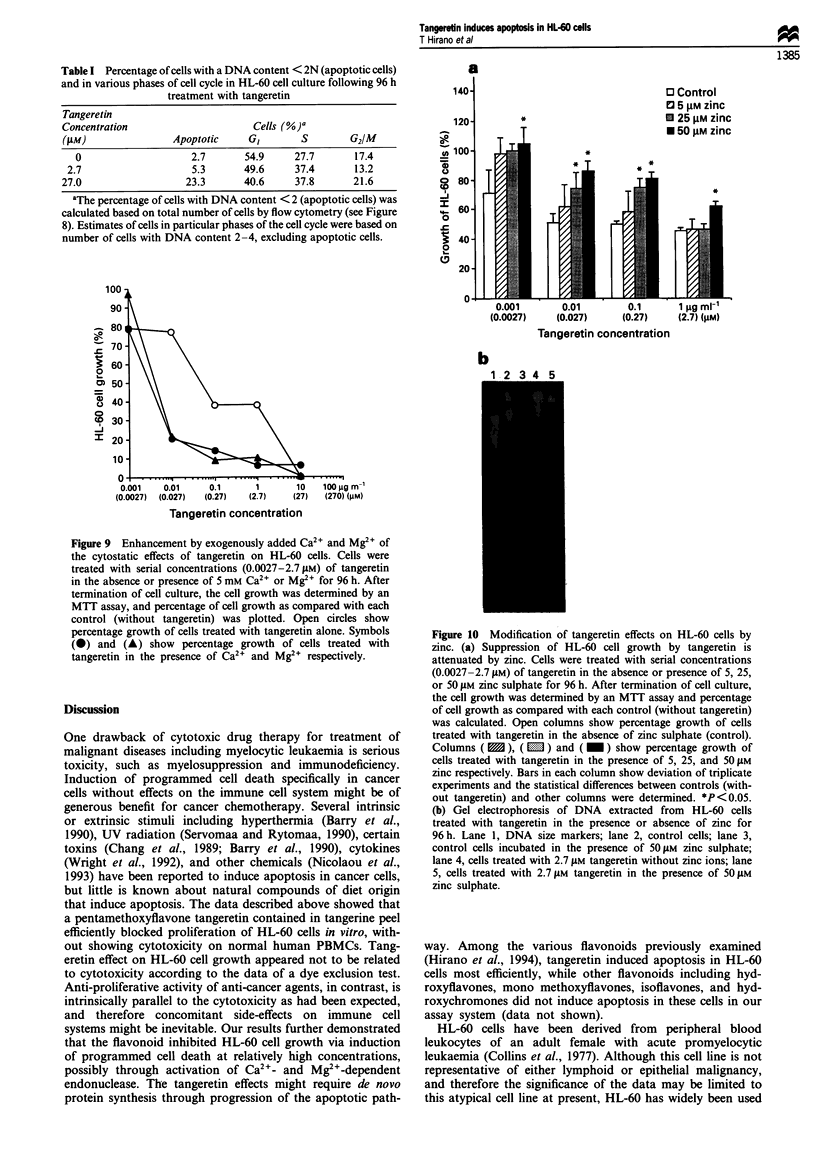
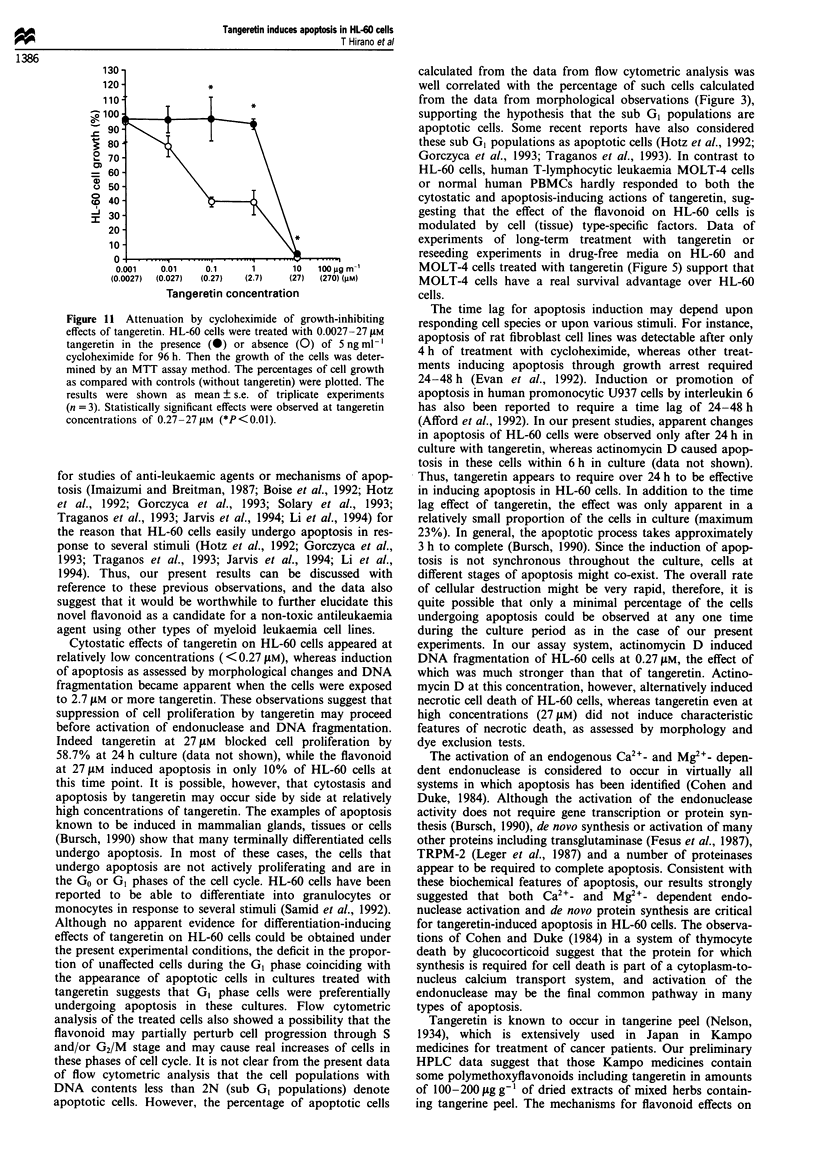
Certain anti-cancer agents are known to induce apoptosis in human tumour cells. However, these agents are intrinsically cytotoxic against cells of normal tissue origin, including myelocytes and immunocytes. Here we show that a naturally occurring flavone of citrus origin, tangeretin (5,6,7,8,4'-pentamethoxyflavone), induces apoptosis in human promyelocytic leukaemia HL-60 cells, whereas the flavone showed no cytotoxicity against human peripheral blood mononuclear cells (PBMCs). The growth of HL-60 cells in vitro assessed by [3H]thymidine incorporation or tetrazolium crystal formation was strongly suppressed in the presence of tangeretin; the IC50 values range between 0.062 and 0.173 microM. Apoptosis of HL-60 cells, assessed by cell morphology and DNA fragmentation, was demonstrated in the presence of > 2.7 microM tangeretin. Flow cytometric analysis of tangeretin-treated HL-60 cells also demonstrated apoptotic cells with low DNA content and showed a decrease of G1 cells and a concomitant increase of S and/or G2/M cells. Apoptosis was evident after 24 h of incubation with tangeretin, and the tangeretin effect as assessed by DNA fragmentation or growth inhibition was significantly attenuated in the presence of Zn2+, which is known to inhibit Ca(2+)-dependent endonuclease activity. Ca2+ and Mg2+, in contrast, promoted the effect of tangeretin. Cycloheximide significantly decreased the tangeretin effect on HL-60 cell growth, suggesting that protein synthesis is required for flavonoid-induced apoptosis. Tangeretin showed no cytotoxicity against either HL-60 cells or mitogen-activated PBMCs even at high concentration (27 microM) as determined by a dye exclusion test. Moreover, the flavonoid was less effective on growth of human T-lymphocytic leukaemia MOLT-4 cells or on blastogenesis of PBMCs. These results suggest that tangeretin inhibits growth of HL-60 cells in vitro, partially through induction of apoptosis, without causing serious side-effects on immune cells.
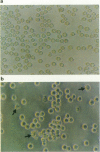
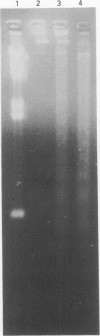
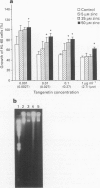
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H. Does fiber-rich food containing animal lignan precursors protect against both colon and breast cancer? An extension of the "fiber hypothesis". Gastroenterology. 1984 Apr;86(4):761–764. [PubMed] [Google Scholar]
- Adlercreutz H., Fotsis T., Heikkinen R., Dwyer J. T., Woods M., Goldin B. R., Gorbach S. L. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet. 1982 Dec 11;2(8311):1295–1299. doi: 10.1016/s0140-6736(82)91507-0. [DOI] [PubMed] [Google Scholar]
- Afford S. C., Pongracz J., Stockley R. A., Crocker J., Burnett D. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J Biol Chem. 1992 Oct 25;267(30):21612–21616. [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Kerrigan D., Sarang M., Pommier Y. Cell death induced by topoisomerase inhibitors. Role of calcium in mammalian cells. Biochem Pharmacol. 1991 Jun 21;42(1):77–85. doi: 10.1016/0006-2952(91)90683-v. [DOI] [PubMed] [Google Scholar]
- Bissery M. C., Valeriote F. A., Chabot G. G., Crissman J. D., Yost C., Corbett T. H. Flavone acetic acid (NSC 347512)-induced DNA damage in Glasgow osteogenic sarcoma in vivo. Cancer Res. 1988 Mar 1;48(5):1279–1285. [PubMed] [Google Scholar]
- Boise L. H., Grant S., Westin E. H. Altered expression of cell cycle related genes including c-myb in a subclone of HL-60 that exhibits reversible differentiation. Cell Growth Differ. 1992 Jan;3(1):53–61. [PubMed] [Google Scholar]
- Bursch W., Kleine L., Tenniswood M. The biochemistry of cell death by apoptosis. Biochem Cell Biol. 1990 Sep;68(9):1071–1074. doi: 10.1139/o90-160. [DOI] [PubMed] [Google Scholar]
- Chang M. P., Baldwin R. L., Bruce C., Wisnieski B. J. Second cytotoxic pathway of diphtheria toxin suggested by nuclease activity. Science. 1989 Dec 1;246(4934):1165–1168. doi: 10.1126/science.2531465. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Compton M. M. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992 Sep;11(2):105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Dive C., Hickman J. A. Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer. 1991 Jul;64(1):192–196. doi: 10.1038/bjc.1991.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. M., Raffauf R. F., Le Quesne P. W. Antineoplastic activity and cytotoxicity of flavones, isoflavones, and flavanones. J Nat Prod. 1979 Jan-Feb;42(1):85–91. doi: 10.1021/np50001a002. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fesus L., Thomazy V., Falus A. Induction and activation of tissue transglutaminase during programmed cell death. FEBS Lett. 1987 Nov 16;224(1):104–108. doi: 10.1016/0014-5793(87)80430-1. [DOI] [PubMed] [Google Scholar]
- Fotsis T., Pepper M., Adlercreutz H., Fleischmann G., Hase T., Montesano R., Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Ardelt B., Traganos F., Darzynkiewicz Z. The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res. 1993 Jul 1;53(13):3186–3192. [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Hirano T., Fukuoka K., Oka K., Naito T., Hosaka K., Mitsuhashi H., Matsumoto Y. Antiproliferative activity of mammalian lignan derivatives against the human breast carcinoma cell line, ZR-75-1. Cancer Invest. 1990;8(6):595–602. doi: 10.3109/07357909009018926. [DOI] [PubMed] [Google Scholar]
- Hirano T., Gotoh M., Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55(13):1061–1069. doi: 10.1016/0024-3205(94)00641-5. [DOI] [PubMed] [Google Scholar]
- Hirano T., Oka K., Akiba M. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75-1. Res Commun Chem Pathol Pharmacol. 1989 Apr;64(1):69–78. [PubMed] [Google Scholar]
- Hirano T., Oka K., Kawashima E., Akiba M. Effects of synthetic and naturally occurring flavonoids on mitogen-induced proliferation of human peripheral-blood lymphocytes. Life Sci. 1989;45(15):1407–1411. doi: 10.1016/0024-3205(89)90028-3. [DOI] [PubMed] [Google Scholar]
- Hotz M. A., Del Bino G., Lassota P., Traganos F., Darzynkiewicz Z. Cytostatic and cytotoxic effects of fostriecin on human promyelocytic HL-60 and lymphocytic MOLT-4 leukemic cells. Cancer Res. 1992 Mar 15;52(6):1530–1535. [PubMed] [Google Scholar]
- Imaizumi M., Breitman T. R. Retinoic acid-induced differentiation of the human promyelocytic leukemia cell line, HL-60, and fresh human leukemia cells in primary culture: a model for differentiation inducing therapy of leukemia. Eur J Haematol. 1987 Apr;38(4):289–302. doi: 10.1111/j.1600-0609.1987.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Jarvis W. D., Turner A. J., Povirk L. F., Traylor R. S., Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res. 1994 Apr 1;54(7):1707–1714. [PubMed] [Google Scholar]
- Kuriki Y., Racker E. Inhibition of (Na+, K+)adenosine triphosphatase and its partial reactions by quercetin. Biochemistry. 1976 Nov 16;15(23):4951–4956. doi: 10.1021/bi00668a001. [DOI] [PubMed] [Google Scholar]
- Li X., Traganos F., Darzynkiewicz Z. Simultaneous analysis of DNA replication and apoptosis during treatment of HL-60 cells with camptothecin and hyperthermia and mitogen stimulation of human lymphocytes. Cancer Res. 1994 Aug 15;54(16):4289–4293. [PubMed] [Google Scholar]
- Léger J. G., Montpetit M. L., Tenniswood M. P. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987 Aug 31;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- Matsukawa Y., Marui N., Sakai T., Satomi Y., Yoshida M., Matsumoto K., Nishino H., Aoike A. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993 Mar 15;53(6):1328–1331. [PubMed] [Google Scholar]
- Nicolaou K. C., Stabila P., Esmaeli-Azad B., Wrasidlo W., Hiatt A. Cell-specific regulation of apoptosis by designed enediynes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3142–3146. doi: 10.1073/pnas.90.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samid D., Shack S., Sherman L. T. Phenylacetate: a novel nontoxic inducer of tumor cell differentiation. Cancer Res. 1992 Apr 1;52(7):1988–1992. [PubMed] [Google Scholar]
- Sargent J. M., Taylor C. G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer. 1989 Aug;60(2):206–210. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servomaa K., Rytömaa T. UV light and ionizing radiations cause programmed death of rat chloroleukaemia cells by inducing retropositions of a mobile DNA element (L1Rn). Int J Radiat Biol. 1990 Feb;57(2):331–343. doi: 10.1080/09553009014552441. [DOI] [PubMed] [Google Scholar]
- Solary E., Bertrand R., Kohn K. W., Pommier Y. Differential induction of apoptosis in undifferentiated and differentiated HL-60 cells by DNA topoisomerase I and II inhibitors. Blood. 1993 Mar 1;81(5):1359–1368. [PubMed] [Google Scholar]
- Suolinna E. M., Buchsbaum R. N., Racker E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975 Jul;35(7):1865–1872. [PubMed] [Google Scholar]
- Traganos F., Kapuscinski J., Gong J., Ardelt B., Darzynkiewicz R. J., Darzynkiewicz Z. Caffeine prevents apoptosis and cell cycle effects induced by camptothecin or topotecan in HL-60 cells. Cancer Res. 1993 Oct 1;53(19):4613–4618. [PubMed] [Google Scholar]
- Verma A. K., Johnson J. A., Gould M. N., Tanner M. A. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988 Oct 15;48(20):5754–5758. [PubMed] [Google Scholar]
- Wright S. C., Kumar P., Tam A. W., Shen N., Varma M., Larrick J. W. Apoptosis and DNA fragmentation precede TNF-induced cytolysis in U937 cells. J Cell Biochem. 1992 Apr;48(4):344–355. doi: 10.1002/jcb.240480403. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. The biology of cell death in tumours. Anticancer Res. 1985 Jan-Feb;5(1):131–136. [PubMed] [Google Scholar]
- Yanagihara K., Ito A., Toge T., Numoto M. Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 1993 Dec 1;53(23):5815–5821. [PubMed] [Google Scholar]