Abstract
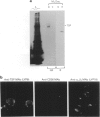
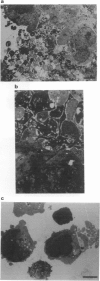
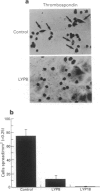
In this study we have investigated the role of thrombospondin (TSP) as a possible ligand playing a key role in human M3Da. melanoma cell interaction with platelets and in tumour growth. TSP is secreted (80 +/- 6 ng TSP 10(-6) cells) and bound to the surface of M3Da. cells via receptors different from CD36, as shown by biosynthetic labelling and immunofluorescence studies. The levels of TSP binding to M3Da. cells evaluated by binding studies, using an anti-TSP monoclonal antibody (MAb) (LYP8), shows 367,000 +/- 58,000 (mean +/- s.d.) LYP8 binding sites per cell with a dissociation constant (Kd) of 67 nM. TSP binding to M3Da. cells shows 400,000 +/- 50,000 TSP binding sites per cell with a Kd of 10 nM. The capacity of anti-TSP MAb (LYP8) to inhibit M3Da.-platelet interactions was followed on an aggregometer and evaluated by electron microscopy studies. The biological role of TSP binding to M3Da. cells was investigated by implanting subcutaneously the M3Da. cell line in nude mice and following the size and time of in vivo tumour growth. Reducing the availability or the functional level of TSP by using an anti-TSP MAb (LYP8) resulted in a significant decrease in platelet aggregates interacting with M3Da. melanoma cells. Using an enzyme-linked immunosorbent assay, purified alpha nu beta 3 was shown to bind TSP. Moreover, LYP8-coated M3Da. cells showed a reduced capacity to form tumours in vivo. M3Da. cells were observed to attach and spread on human platelet TSP-coated plastic wells. This attachment by M3Da. cells was inhibited in a similar way by LYP8 and an anti-alpha nu beta 3 MAb (LYP18). The results obtained in this study show that TSP secreted and bound to the surface of a human melanoma cell line (M3Da.) acts as a link between aggregated platelets and the M3Da. cell surface. Moreover, these results shows that TSP can modulate tumour growth in vivo. Reagents such as MAbs directed against TSP and peptides derived from TSP could not only be used as a new therapeutic approach in the control of tumour metastasis of melanoma, but may also contribute to elucidation of the role of TSP in cancer biology.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbadia Z., Clezardin P., Serre C. M., Amiral J., Delmas P. D. Thrombospondin (TSP1) mediates in vitro proliferation of human MG-63 osteoblastic cells induced by alpha-thrombin. FEBS Lett. 1993 Aug 30;329(3):341–346. doi: 10.1016/0014-5793(93)80250-x. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch A. S., Silbiger S., Heimer E., Nachman R. L. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Tepler J., Silbiger S., Nachman R. L. Cellular attachment to thrombospondin. Cooperative interactions between receptor systems. J Biol Chem. 1991 Jan 25;266(3):1740–1745. [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992 Nov;6(14):3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- Boukerche H., Berthier-Vergnes O., Bailly M., Doré J. F., Leung L. L., McGregor J. L. A monoclonal antibody (LYP18) directed against the blood platelet glycoprotein IIb/IIIa complex inhibits human melanoma growth in vivo. Blood. 1989 Aug 15;74(3):909–912. [PubMed] [Google Scholar]
- Boukerche H., Berthier-Vergnes O., Penin F., Tabone E., Lizard G., Bailly M., McGregor J. L. Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. Br J Haematol. 1994 Aug;87(4):763–772. doi: 10.1111/j.1365-2141.1994.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Boukerche H., Berthier-Vergnes O., Tabone E., Doré J. F., Leung L. L., McGregor J. L. Platelet-melanoma cell interaction is mediated by the glycoprotein IIb-IIIa complex. Blood. 1989 Aug 1;74(2):658–663. [PubMed] [Google Scholar]
- Boukerche H., McGregor J. L. Characterization of an anti-thrombospondin monoclonal antibody (P8) that inhibits human blood platelet functions. Normal binding of P8 to thrombin-activated Glanzmann thrombasthenic platelets. Eur J Biochem. 1988 Jan 15;171(1-2):383–392. doi: 10.1111/j.1432-1033.1988.tb13802.x. [DOI] [PubMed] [Google Scholar]
- Castle V., Varani J., Fligiel S., Prochownik E. V., Dixit V. Antisense-mediated reduction in thrombospondin reverses the malignant phenotype of a human squamous carcinoma. J Clin Invest. 1991 Jun;87(6):1883–1888. doi: 10.1172/JCI115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catimel B., Leung L., el Ghissasi H., Mercier N., McGregor J. Human platelet glycoprotein IIIb binds to thrombospondin fragments bearing the C-terminal region, and/or the type I repeats (CSVTCG motif), but not to the N-terminal heparin-binding region. Biochem J. 1992 May 15;284(Pt 1):231–236. doi: 10.1042/bj2840231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Galvin N. J., O'Rourke K. M., Frazier W. A. Monoclonal antibodies that recognize calcium-dependent structures of human thrombospondin. Characterization and mapping of their epitopes. J Biol Chem. 1986 Feb 5;261(4):1962–1968. [PubMed] [Google Scholar]
- Dvorak H. F., Nagy J. A., Dvorak A. M. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cells. 1991 Mar;3(3):77–85. [PubMed] [Google Scholar]
- Felding-Habermann B., Mueller B. M., Romerdahl C. A., Cheresh D. A. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992 Jun;89(6):2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Galanti N., Johnson T., Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973 May;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Jacubovich R., Cabrillat H., Dore J. F. Natural resistance to xenografts of human malignant melanoma cell lines in nude mice. Absence of demonstrated role of NK activities. Exp Cell Biol. 1984;52(1-2):48–52. [PubMed] [Google Scholar]
- Karczewski J., Knudsen K. A., Smith L., Murphy A., Rothman V. L., Tuszynski G. P. The interaction of thrombospondin with platelet glycoprotein GPIIb-IIIa. J Biol Chem. 1989 Dec 15;264(35):21322–21326. [PubMed] [Google Scholar]
- Kehrel B., Kronenberg A., Rauterberg J., Niesing-Bresch D., Niehues U., Kardoeus J., Schwippert B., Tschöpe D., van de Loo J., Clemetson K. J. Platelets deficient in glycoprotein IIIb aggregate normally to collagens type I and III but not to collagen type V. Blood. 1993 Dec 1;82(11):3364–3370. [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L. Role of thrombospondin in platelet aggregation. J Clin Invest. 1984 Nov;74(5):1764–1772. doi: 10.1172/JCI111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Goodman L. V., Dixit V. M. Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988 Feb;106(2):415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. F., Nesbitt S. A., Helfrich M. H., Horton M. A., Polakova K., Hart I. R. Integrin expression in human melanoma cell lines: heterogeneity of vitronectin receptor composition and function. Int J Cancer. 1991 Dec 2;49(6):924–931. doi: 10.1002/ijc.2910490621. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Catimel B., Parmentier S., Clezardin P., Dechavanne M., Leung L. L. Rapid purification and partial characterization of human platelet glycoprotein IIIb. Interaction with thrombospondin and its role in platelet aggregation. J Biol Chem. 1989 Jan 5;264(1):501–506. [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Newman P. J., McEver R. P., Doers M. P., Kunicki T. J. Synergistic action of two murine monoclonal antibodies that inhibit ADP-induced platelet aggregation without blocking fibrinogen binding. Blood. 1987 Feb;69(2):668–676. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988 Nov 15;948(2):175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Nierodzik M. L., Plotkin A., Kajumo F., Karpatkin S. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest. 1991 Jan;87(1):229–236. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B. Fibrinogen receptor exposure and aggregation of human blood platelets produced by ADP and chilling. Blood. 1981 Apr;57(4):663–670. [PubMed] [Google Scholar]
- Riser B. L., Varani J., O'Rourke K., Dixit V. M. Thrombospondin binding by human squamous carcinoma and melanoma cells: relationship to biological activity. Exp Cell Res. 1988 Feb;174(2):319–329. doi: 10.1016/0014-4827(88)90303-5. [DOI] [PubMed] [Google Scholar]
- Roberts D. D. Interactions of thrombospondin with sulfated glycolipids and proteoglycans of human melanoma cells. Cancer Res. 1988 Dec 1;48(23):6785–6793. [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Baird M., Lo S. K., Yesner L. M. Sense and antisense cDNA transfection of CD36 (glycoprotein IV) in melanoma cells. Role of CD36 as a thrombospondin receptor. J Biol Chem. 1992 Aug 15;267(23):16607–16612. [PubMed] [Google Scholar]
- Tolsma S. S., Volpert O. V., Good D. J., Frazier W. A., Polverini P. J., Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993 Jul;122(2):497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Gasic T. B., Rothman V. L., Knudsen K. A., Gasic G. J. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res. 1987 Aug 1;47(15):4130–4133. [PubMed] [Google Scholar]
- Tuszynski G. P., Karczewski J., Smith L., Murphy A., Rothman V. L., Knudsen K. A. The GPIIB-IIIa-like complex may function as a human melanoma cell adhesion receptor for thrombospondin. Exp Cell Res. 1989 Jun;182(2):473–481. doi: 10.1016/0014-4827(89)90251-6. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Kowalska M. A. Thrombospondin-induced adhesion of human platelets. J Clin Invest. 1991 Apr;87(4):1387–1394. doi: 10.1172/JCI115144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V. L., Deutch A. H., Hamilton B. K., Eyal J. Biological activities of peptides and peptide analogues derived from common sequences present in thrombospondin, properdin, and malarial proteins. J Cell Biol. 1992 Jan;116(1):209–217. doi: 10.1083/jcb.116.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Riser B. L., Hughes L. A., Carey T. E., Fligiel S. E., Dixit V. M. Characterization of thrombospondin synthesis, secretion and cell surface expression by human tumor cells. Clin Exp Metastasis. 1989 May-Jun;7(3):265–276. doi: 10.1007/BF01753679. [DOI] [PubMed] [Google Scholar]
- Walz D. A. Thrombospondin as a mediator of cancer cell adhesion in metastasis. Cancer Metastasis Rev. 1992 Nov;11(3-4):313–324. doi: 10.1007/BF01307185. [DOI] [PubMed] [Google Scholar]
- Yabkowitz R., Dixit V. M. Human carcinoma cells bind thrombospondin through a Mr 80,000/105,000 receptor. Cancer Res. 1991 Jul 15;51(14):3648–3656. [PubMed] [Google Scholar]