Abstract
The need for sample size calculations is briefly reviewed: many of the arguments against small trials are already well known, and we only cursorily repeat them in passing. Problems that arise in the estimation of sample size are then discussed, with particular reference to survival studies. However, most of the issues which we discuss are equally applicable to other types of study. Finally, prognostic factor analysis designs are discussed, since this is another area in which experience shows that far too many studies are of an inadequate size and yield misleading results.
Full text
PDF




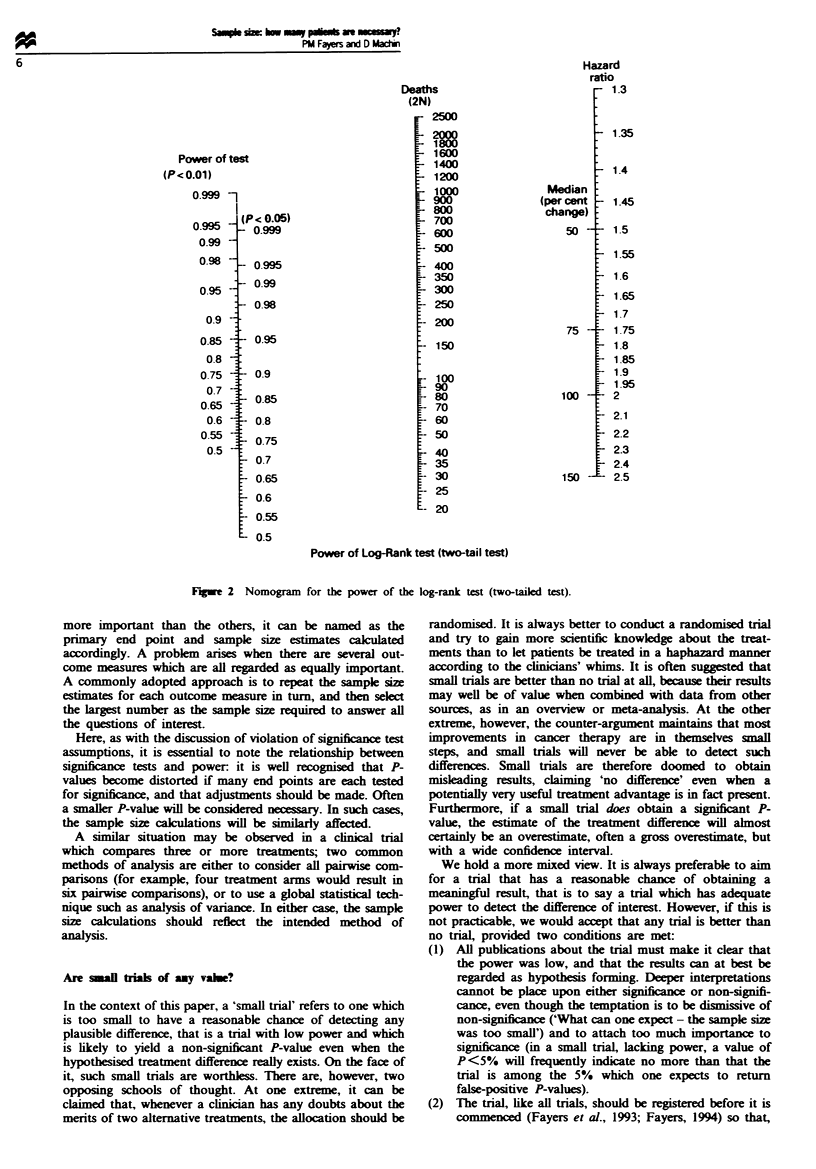



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman D. G., Gore S. M., Gardner M. J., Pocock S. J. Statistical guidelines for contributors to medical journals. Br Med J (Clin Res Ed) 1983 May 7;286(6376):1489–1493. doi: 10.1136/bmj.286.6376.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D. G. Statistics and ethics in medical research: III How large a sample? Br Med J. 1980 Nov 15;281(6251):1336–1338. doi: 10.1136/bmj.281.6251.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers T. C., Matta R. J., Smith H., Jr, Kunzler A. M. Evidence favoring the use of anticoagulants in the hospital phase of acute myocardial infarction. N Engl J Med. 1977 Nov 17;297(20):1091–1096. doi: 10.1056/NEJM197711172972004. [DOI] [PubMed] [Google Scholar]
- Dickersin K., Chan S., Chalmers T. C., Sacks H. S., Smith H., Jr Publication bias and clinical trials. Control Clin Trials. 1987 Dec;8(4):343–353. doi: 10.1016/0197-2456(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Diehl L. F., Perry D. J. A comparison of randomized concurrent control groups with matched historical control groups: are historical controls valid? J Clin Oncol. 1986 Jul;4(7):1114–1120. doi: 10.1200/JCO.1986.4.7.1114. [DOI] [PubMed] [Google Scholar]
- Fayers P. M., Armitage T. Towards an international register of cancer trials: the UKCCCR register of U.K. trials. Eur J Cancer. 1993;29A(6):907–912. doi: 10.1016/s0959-8049(05)80436-8. [DOI] [PubMed] [Google Scholar]
- Fayers P. M., Cook P. A., Machin D., Donaldson N., Whitehead J., Ritchie A., Oliver R. T., Yuen P. On the development of the Medical Research Council trial of alpha-interferon in metastatic renal carcinoma. Urological Working Party Renal Carcinoma Subgroup. Stat Med. 1994 Nov 15;13(21):2249–2260. doi: 10.1002/sim.4780132106. [DOI] [PubMed] [Google Scholar]
- Fielding L. P., Fenoglio-Preiser C. M., Freedman L. S. The future of prognostic factors in outcome prediction for patients with cancer. Cancer. 1992 Nov 1;70(9):2367–2377. doi: 10.1002/1097-0142(19921101)70:9<2367::aid-cncr2820700927>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Freedman L. S. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982 Apr-Jun;1(2):121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- Freedman L. S. The size of clinical trials in cancer research--what are the current needs? Medical Research Council Cancer Therapy Committee. Br J Cancer. 1989 Mar;59(3):396–400. doi: 10.1038/bjc.1989.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman J. A., Chalmers T. C., Smith H., Jr, Kuebler R. R. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. N Engl J Med. 1978 Sep 28;299(13):690–694. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Machin D., Campbell M. J. Use of check lists in assessing the statistical content of medical studies. Br Med J (Clin Res Ed) 1986 Mar 22;292(6523):810–812. doi: 10.1136/bmj.292.6523.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehan E. A. The evaluation of therapies: historical control studies. Stat Med. 1984 Oct-Dec;3(4):315–324. doi: 10.1002/sim.4780030405. [DOI] [PubMed] [Google Scholar]
- Goodman S. N. A comment on replication, p-values and evidence. Stat Med. 1992 May;11(7):875–879. doi: 10.1002/sim.4780110705. [DOI] [PubMed] [Google Scholar]
- Goodman S. N., Berlin J. A. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994 Aug 1;121(3):200–206. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- Harrell F. E., Jr, Lee K. L., Matchar D. B., Reichert T. A. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat Rep. 1985 Oct;69(10):1071–1077. [PubMed] [Google Scholar]
- Haybittle J. L., Alcock C. J., Fowler J. F., Hopewell J. W., Rezvani M., Wiernik G. Recruitment, follow-up and analysis times in clinical trials of cancer treatment: a case study. Br J Cancer. 1990 Oct;62(4):687–691. doi: 10.1038/bjc.1990.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micciolo R., Valagussa P., Marubini E. The use of historical controls in breast cancer. An assessment in three consecutive trials. Control Clin Trials. 1985 Dec;6(4):259–270. doi: 10.1016/0197-2456(85)90102-3. [DOI] [PubMed] [Google Scholar]
- Newcombe R. G. Towards a reduction in publication bias. Br Med J (Clin Res Ed) 1987 Sep 12;295(6599):656–659. doi: 10.1136/bmj.295.6599.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock S. J. The combination of randomized and historical controls in clinical trials. J Chronic Dis. 1976 Mar;29(3):175–188. doi: 10.1016/0021-9681(76)90044-8. [DOI] [PubMed] [Google Scholar]
- Sacks H. S., Chalmers T. C., Smith H., Jr Sensitivity and specificity of clinical trials. Randomized v historical controls. Arch Intern Med. 1983 Apr;143(4):753–755. [PubMed] [Google Scholar]
- Simes R. J. Publication bias: the case for an international registry of clinical trials. J Clin Oncol. 1986 Oct;4(10):1529–1541. doi: 10.1200/JCO.1986.4.10.1529. [DOI] [PubMed] [Google Scholar]
- Simon R., Altman D. G. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994 Jun;69(6):979–985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter D. J., Freedman L. S. A predictive approach to selecting the size of a clinical trial, based on subjective clinical opinion. Stat Med. 1986 Jan-Feb;5(1):1–13. doi: 10.1002/sim.4780050103. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter D. J., Freedman L. S., Parmar M. K. Applying Bayesian ideas in drug development and clinical trials. Stat Med. 1993 Aug;12(15-16):1501–1517. doi: 10.1002/sim.4780121516. [DOI] [PubMed] [Google Scholar]
- Wittes J., Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med. 1990 Jan-Feb;9(1-2):65–72. doi: 10.1002/sim.4780090113. [DOI] [PubMed] [Google Scholar]
- Yusuf S., Collins R., Peto R. Why do we need some large, simple randomized trials? Stat Med. 1984 Oct-Dec;3(4):409–422. doi: 10.1002/sim.4780030421. [DOI] [PubMed] [Google Scholar]


