Abstract
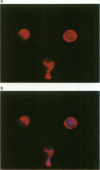
The intracellular distribution of glutathione (GSH) was measured by a quantitative image cytometry method, using the sulphydryl-reactive agent mercury orange. This readily forms fluorescent adducts with GSH and other non-protein sulphydryls (NPSH), but reacts much more slowly with protein sulphydryls. Under optimum staining conditions mean integrated mercury orange fluorescence per cell was closely correlated with a standard biochemical assay for GSH. Use of the DNA dye DAPI as a counterstain allowed measurement of nuclear NPSH. The mean nuclear-cytoplasmic ratio was 0.57 +/- 0.05. Isolation of nuclei under aqueous conditions resulted in the loss of approximately 90% of mercury orange fluorescence, compared with nuclear fluorescence from intact cells, suggesting that background labelling of protein sulphydryls or other macromolecules is low. Depletion of GSH with N-ethylmaleimide or diethylmaleate decreased mercury orange fluorescence in the nucleus and cytoplasm to a similar extent. In contrast, mercury orange fluorescence in the nucleus was much more resistant to DL-buthionine-S,R-sulphoximine (BSO) depletion than that in the cytoplasm. This finding is compatible with a distinct pool of GSH in the nucleus that is comparatively resistant to BSO depletion. Alternatively, the retention of fluorescence in the nucleus following GSH depletion by BSO treatment might be due to accumulation of cysteine. These findings have implications for cancer treatment since the level of NPSH in the nucleus might be a more important determinant of resistance to DNA-damaging agents than that in cytoplasm. The image cytometry method described here is quantitative, allows a measure of tumour cell heterogeneity and can be applied to small biopsy samples obtained by fine-needle aspiration. Thus it appears suitable for prospective clinical studies in cancer patients, and for monitoring the effects of GSH-depleting agents used as adjuncts to cancer chemotherapy or radiotherapy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Schiefer M. A., Murphy M. P., Howell S. B. Enhanced potentiation of cisplatin cytotoxicity in human ovarian carcinoma cells by prolonged glutathione depletion. Chem Biol Interact. 1988;65(1):51–58. doi: 10.1016/0009-2797(88)90030-0. [DOI] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Asghar K., Reddy B. G., Krishna G. Histochemical localization of glutathione in tissues. J Histochem Cytochem. 1975 Oct;23(10):774–779. doi: 10.1177/23.10.53246. [DOI] [PubMed] [Google Scholar]
- Astor M. B. Radiobiological studies with a series of human cell lines of varying glutathione content. Br J Radiol. 1984 Aug;57(680):717–722. doi: 10.1259/0007-1285-57-680-717. [DOI] [PubMed] [Google Scholar]
- Bailey H. H., Mulcahy R. T., Tutsch K. D., Arzoomanian R. Z., Alberti D., Tombes M. B., Wilding G., Pomplun M., Spriggs D. R. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol. 1994 Jan;12(1):194–205. doi: 10.1200/JCO.1994.12.1.194. [DOI] [PubMed] [Google Scholar]
- Barranco S. C., Townsend C. M., Jr, Weintraub B., Beasley E. G., MacLean K. K., Shaeffer J., Liu N. H., Schellenberg K. Changes in glutathione content and resistance to anticancer agents in human stomach cancer cells induced by treatments with melphalan in vitro. Cancer Res. 1990 Jun 15;50(12):3614–3618. [PubMed] [Google Scholar]
- Bellomo G., Vairetti M., Stivala L., Mirabelli F., Richelmi P., Orrenius S. Demonstration of nuclear compartmentalization of glutathione in hepatocytes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4412–4416. doi: 10.1073/pnas.89.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaglow J. E., Varnes M. E., Clark E. P., Epp E. R. The role of thiols in cellular response to radiation and drugs. Radiat Res. 1983 Sep;95(3):437–455. [PubMed] [Google Scholar]
- Britten R. A., Green J. A., Broughton C., Browning P. G., White R., Warenius H. M. The relationship between nuclear glutathione levels and resistance to melphalan in human ovarian tumour cells. Biochem Pharmacol. 1991 Feb 15;41(4):647–649. doi: 10.1016/0006-2952(91)90642-i. [DOI] [PubMed] [Google Scholar]
- Briviba K., Fraser G., Sies H., Ketterer B. Distribution of the monochlorobimane-glutathione conjugate between nucleus and cytosol in isolated hepatocytes. Biochem J. 1993 Sep 15;294(Pt 3):631–633. doi: 10.1042/bj2940631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. A., Pass H. I., Iype S. N., Friedman N., DeGraff W., Russo A., Mitchell J. B. Cellular glutathione and thiol measurements from surgically resected human lung tumor and normal lung tissue. Cancer Res. 1991 Aug 15;51(16):4287–4294. [PubMed] [Google Scholar]
- Dusre L., Mimnaugh E. G., Myers C. E., Sinha B. K. Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells. Cancer Res. 1989 Feb 1;49(3):511–515. [PubMed] [Google Scholar]
- Edgren M., Révész L. Compartmentalised depletion of glutathione in cells treated with buthionine sulphoximine. Br J Radiol. 1987 Jul;60(715):723–724. doi: 10.1259/0007-1285-60-715-723. [DOI] [PubMed] [Google Scholar]
- Eyer P., Podhradský D. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman's reagent. Anal Biochem. 1986 Feb 15;153(1):57–66. doi: 10.1016/0003-2697(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Hansson J., Edgren M., Ehrsson H., Ringborg U., Nilsson B. Effect of D,L-buthionine-S,R-sulfoximine on cytotoxicity and DNA cross-linking induced by bifunctional DNA-reactive cytostatic drugs in human melanoma cells. Cancer Res. 1988 Jan 1;48(1):19–26. [PubMed] [Google Scholar]
- Hosking L. K., Whelan R. D., Shellard S. A., Bedford P., Hill B. T. An evaluation of the role of glutathione and its associated enzymes in the expression of differential sensitivities to antitumour agents shown by a range of human tumour cell lines. Biochem Pharmacol. 1990 Oct 15;40(8):1833–1842. doi: 10.1016/0006-2952(90)90364-q. [DOI] [PubMed] [Google Scholar]
- Hulbert P. B., Yakubu S. I. Monobromobimane: a substrate for the fluorimetric assay of glutathione transferase. J Pharm Pharmacol. 1983 Jun;35(6):384–386. doi: 10.1111/j.2042-7158.1983.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Jevtović-Todorović V., Guenthner T. M. Depletion of a discrete nuclear glutathione pool by oxidative stress, but not by buthionine sulfoximine. Correlation with enhanced alkylating agent cytotoxicity to human melanoma cells in vitro. Biochem Pharmacol. 1992 Oct 6;44(7):1383–1393. doi: 10.1016/0006-2952(92)90540-y. [DOI] [PubMed] [Google Scholar]
- Jocelyn P. C., Cronshaw A. Properties of mitochondria treated with 1-chloro-2,4-dinitrobenzene. Biochem Pharmacol. 1985 May 1;34(9):1588–1590. doi: 10.1016/0006-2952(85)90706-3. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kudo H., Mio T., Kokunai T., Tamaki N., Sumino K., Matsumoto S. Quantitative analysis of glutathione in human brain tumors. J Neurosurg. 1990 Apr;72(4):610–615. doi: 10.3171/jns.1990.72.4.0610. [DOI] [PubMed] [Google Scholar]
- Larrauri A., López P., Gómez-Lechón M. J., Castell J. V. A cytochemical stain for glutathione in rat hepatocytes cultured on plastic. J Histochem Cytochem. 1987 Feb;35(2):271–274. doi: 10.1177/35.2.2432118. [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Siemann D. W. Isolation by flow cytometry of a human ovarian tumor cell subpopulation exhibiting a high glutathione content phenotype and increased resistance to adriamycin. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1315–1319. doi: 10.1016/0360-3016(89)90306-4. [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Vessey A., Rofstad E., Siemann D. W., Sutherland R. M. Heterogeneity of glutathione content in human ovarian cancer. Cancer Res. 1989 Oct 1;49(19):5244–5248. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mistry P., Harrap K. R. Historical aspects of glutathione and cancer chemotherapy. Pharmacol Ther. 1991;49(1-2):125–132. doi: 10.1016/0163-7258(91)90026-i. [DOI] [PubMed] [Google Scholar]
- Newton G. L., Dorian R., Fahey R. C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- Perry R. R., Mazetta J. A., Levin M., Barranco S. C. Glutathione levels and variability in breast tumors and normal tissue. Cancer. 1993 Aug 1;72(3):783–787. doi: 10.1002/1097-0142(19930801)72:3<783::aid-cncr2820720325>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Reed D. J. Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- Russo A., Carmichael J., Friedman N., DeGraff W., Tochner Z., Glatstein E., Mitchell J. B. The roles of intracellular glutathione in antineoplastic chemotherapy. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1347–1354. doi: 10.1016/0360-3016(86)90169-0. [DOI] [PubMed] [Google Scholar]
- Révész L. The role of endogenous thiols in intrinsic radioprotection. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Apr;47(4):361–368. [PubMed] [Google Scholar]
- Sandström B. E., Marklund S. L. Effects of variation in glutathione peroxidase activity on DNA damage and cell survival in human cells exposed to hydrogen peroxide and t-butyl hydroperoxide. Biochem J. 1990 Oct 1;271(1):17–23. doi: 10.1042/bj2710017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrieve D. C., Bump E. A., Rice G. C. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988 Oct 5;263(28):14107–14114. [PubMed] [Google Scholar]
- Taylor C. W., Yeoman L. C., Daskal I., Busch H. Two-dimensional electrophoresis of proteins of citric acid nuclei prepared with aid of a Tissumizer. Exp Cell Res. 1973 Nov;82(1):215–226. doi: 10.1016/0014-4827(73)90264-4. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tirmenstein M. A., Reed D. J. The glutathione status of rat kidney nuclei following administration of buthionine sulfoximine. Biochem Biophys Res Commun. 1988 Sep 15;155(2):956–961. doi: 10.1016/s0006-291x(88)80589-8. [DOI] [PubMed] [Google Scholar]
- Treumer J., Valet G. Flow-cytometric determination of glutathione alterations in vital cells by o-phthaldialdehyde (OPT) staining. Exp Cell Res. 1986 Apr;163(2):518–524. doi: 10.1016/0014-4827(86)90082-0. [DOI] [PubMed] [Google Scholar]