Abstract
Velocardiofacial syndrome (VCFS), also known as 22q11.2 deletion syndrome, is a neurogenetic disorder that is associated with both learning disabilities and a consistent neuropsychological phenotype, including deficits in executive function, visuospatial perception, and working memory. Anatomic imaging studies have identified significant volumetric reductions in the parietal lobe of individuals with VCFS, but several studies have reported that the frontal lobe is relatively preserved. We used functional magnetic resonance imaging to investigate the neural correlates of non-spatial working memory in 17 youths with VCFS, 10 of their unaffected siblings, and 10 community controls (with the same proportion of learning disabilities as the VCFS youths). Task performance of siblings tended to be more accurate than children with VCFS, who did not differ from community controls. All three study groups recruited parietal regions that were equivalent in location and magnitude. Whereas the sibling group also recruited the dorsolateral prefrontal cortex (DLPFC), Broca’s area, and anterior cingulate, DLPFC activation was absent in the whole brain analyses of children with VCFS and controls. Moreover, the magnitude of frontal activation in VCFS participants was restricted relative to both siblings and controls. These findings suggest that VCFS participants exhibit frontal hypoactivation that is not attributable to performance. In addition, VCFS children and controls (many with idiopathic learning disabilities) appear to rely on phonological rehearsal to hold information on line instead of the DLPFC. Despite previous anatomic MRI reports of preserved frontal lobe volumes in VCFS therefore, these fMRI findings suggest that the frontal component of the distributed network subserving executive function and working memory may be disrupted in youth with this disorder.
Keywords: velocardiofacial syndrome, VCFS, 22q11.2 deletion syndrome, working memory, functional MRI, frontal lobe, parietal lobe
1. Introduction
Velocardiofacial syndrome (VCFS) is a multiple anomaly syndrome caused by a microdeletion of chromosome 22 at the q11.2 band (Driscoll et al., 1992; Scambler et al., 1992). VCFS is associated with a characteristic facial appearance, including a long face, a prominent nose and vertically narrow eyes. The physical phenotype can include palatal, cardiac vascular, renal, limb, spine, and immunologic anomalies (Robin and Shprintzen, 2005; Shprintzen, 2005). Speech and language impairments are very common (Shprintzen, 2005). Individuals with VCFS also display a distinct cognitive phenotype, which includes a wide range of IQs, spanning from the average to the mild/moderate retardation range (Swillen et al., 1997). Nearly all children with VCFS have learning disabilities. Exhibiting a fairly consistent neuropsychological phenotype, those with VCFS typically score relatively low on tests of attention, executive function, emotional processing, sensorimotor processing, visuospatial processing, and working memory (Bearden et al., 2001; Sobin et al., 2004; Van Amelsvoort et al., 2004; Bish et al., 2005).
Working memory is the ability to temporarily sustain and manipulate task related information and is a key component of cognition and executive functioning; working memory undergirds other executive functions such as language production and acquisition, learning, and problem solving (Baddeley, 1992; Gathercole et al., 2004; Nelson, 1995). Previous neuropsychological studies have shown that subjects with VCFS demonstrate relatively lower performance in tasks of working memory, including verbal working memory and visual spatial memory (Baker et al., 2005; Bearden et al., 2001; Lajiness-O’Neill et al., 2005; Sobin et al., 2005).
Relatively few functional imaging studies have investigated working memory in typically developing children and adolescents, and only a subset of those have examined non-spatial working memory. These studies suggest that typically developing children and adolescents recruit a network consisting of the dorsolateral prefrontal cortex and the parietal cortex (Casey et al., 1995; Thomas et al., 1999; Nelson et al., 2000; Klingberg et al., 2002; see review by Dowker, 2006). As typical individuals approach adulthood, this network broadens to include temporal and subcortical regions (Braver et al., 1997; Lewis et al., 2004). The few studies that have focused on the developmental trajectory of brain function have reported that prefrontal and parietal activation increased with age during working memory tasks (Klingberg et al., 2006;Kwon et al., 2002). This is most likely consistent with developmental changes in cortical structure (see Lenroot & Giedd, 2006) that have been described during the past decade. Longitudinal studies have shown that whereas total frontal and parietal gray matter volumes peak in girls at ages 11.0 and 10.2, respectively, and in boys at ages12.1 and 11.8, respectively, the dosolateral prefrontal cortex specifically undergoes a prolonged maturational trajectory, with cortical thickening continuing into late adolescence (Gotgay et al., 2004).
Volumetric MRI studies have shown that many of the regions included within the working memory network are altered in VCFS. Although several volumetric studies based on a semi-automated method of cerebral parcellation suggest that the frontal lobe is relatively preserved in VCFS, we have recently reported preliminary findings based on manual measurements of the frontal lobe that suggest that frontal volumes are altered in VCFS (Kates et al., 2004). Furthermore, reductions in white matter volumes of superior and medial frontal regions have been found in adults with VCFS (Van Amelsvoort et al., 2001). Volumetric data also support the presence of robust deficits in the parietal lobes of individuals with VCFS (Eliez et al., 2000; Kates et al., 2001), and diffusion tensor imaging studies (Barnea-Goraly et al., 2003; Simon et al., 2005) suggest that frontal – parietal connectivity may be disrupted in children with the disorder. However, none of these studies investigated the association between frontal-parietal alterations and deficits in working memory. Conducting an fMRI study of working memory in VCFS is critical to our understanding of the neurophysiology that contributes to the cognitive deficits and learning disabilities that are so well documented.
Insofar as the studies of cognitive deficits and learning disabilities in VCFS have largely been descriptive and global in their approach, one goal of the current study was to determine whether potential disturbances in neural functioning of children with VCFS were due to VCFS per se, or instead to more general CNS dysfunction that may characterize all children with deficits in learning and attention. Several studies (Feinstein et al., 2002; Swillen et al., 2001) suggest that psychiatric and behavioral disorders are not specific to VCFS but instead are associated with overall cognitive dysfunction. However few, if any, studies of neuroanatomy or neurofunction in VCFS have included a cognitively matched control group that would address this question of specificity. This is due, in part, to the methodological risks inherent in recruiting a cognitively matched sample that may include children with significant intellectual disabilities of unknown etiology (making data interpretation difficult). For this reason, we decided not to recruit a control sample whose range of cognitive function matched our VCFS sample. Instead, we chose to recruit a control sample that was cognitively similar to our higher functioning VCFS subjects, most of whom had learning disabilities.
Accordingly, in this study we investigate the neural correlates of non-spatial working memory in children with VCFS, age-matched siblings, and a sample of community controls, many of whom have idiopathic learning disabilities. We expect that children with VCFS will rely on a neural network during a working memory fMRI paradigm that is distinct from both their typically developing siblings and from peers who also exhibit learning disabilities but do not have VCFS.
2. Experimental Procedure
2.1 Subjects
Forty-three children aged 8–15 years originally participated in the study. One control subject, one sibling and seven children with VCFS (57% female) were excluded from analyses due to poor behavioral performance (see section 4.5, analysis of behavioral data). The children with VCFS who were excluded from the study did not differ from VCFS children who were included in FSIQ (p = .41) or age (p = .11).
The final study groups consisted of 17 children with VCFS (7 females, 10 males), 10 siblings of VCFS subjects (5 females, 5 males) and 10 age-matched controls (4 females, 6 males). Children with VCFS were recruited from the Center for the Diagnosis, Treatment, and Study of VCFS at SUNY-Upstate Medical University. All participants with VCFS had 22q11.2 deletions confirmed by FISH (fluorescence in situ hybridization). Siblings of children with VCFS were enrolled if they were within the same age range of the VCFS-affected participants, and if they met the inclusion criteria listed below. Our control participants were recruited from local public schools. In an effort to match our controls to our higher-functioning VCFS participants as closely as possible, we did not exclude controls that displayed learning disabilities or ADHD.
We used two methods to determine if children had learning disabilities: Children were designated as learning disabled 1) if they exhibited a discrepancy of 1.5 standard deviations between their FSIQ and their total composite score on the academic achievement assessment; or 2) if they attained a composite score on the WIAT-II below 85. Our operationalization of learning disabilities was consistent with other definitions (Stanovich and Siegel, 1994) and is based on research demonstrating that IQ-discrepant and low achieving children overlap and are strikingly similar across multiple behavioral and cognitive parameters (Fletcher et al., 2002; Hoskyn and Swanson, 2000; Stuebing et al., 2002).
Children with an identifiable genetic disorder (other than VCFS) and/or children with an identifiable neurological condition (e.g., traumatic brain injury; birth weight under 2500 grams as reported by parent; seizure disorder) that is known to affect cognitive or psychiatric function were excluded from participation. The institutional review board of the Upstate Medical University approved the protocol. Informed consent/assent was obtained from all subjects.
2.2 Psychological Assessment
IQ was assessed with the Wechsler Intelligence Scale for Children – 3rd Edition (Wechsler, 1991). Only full-scale IQ (FSIQ) scores were used for the analyses in this report. Academic achievement was assessed with the Wechsler Individual Achievement Test – 2nd edition (WIAT-II). The WIAT-II (Wechsler, 2001) is an individually administered test of academic achievement, the scores of which are aggregated into four composite scores: reading, mathematics, language, and writing, as well as a total composite score. Only the total composite score was used for the analyses in this report.
2.3 Experimental Design
We administered a two – back, non-spatial working memory task, which consisted of alternating presentations of three experimental blocks and three control blocks. For the experimental blocks of the paradigm, subjects are asked to respond, via a button box (Rowland Institute, Cambridge, MA) when they saw the same letter as two letters back (Fig. 1a). During the control block subjects responded only to the letter “X” (Fig. 1b). Blocks consist of 25 stimuli. The duration of each stimulus was 1250 ms with an interstimulus interval of 1250 ms. Instructions were presented on screen for 7.5 s before each block was shown. For each block, 32% of the trials required a response. Response reaction time was noted. Prior to scanning subjects were trained on the task to the point that they displayed adequate out-of-scanner performance, as defined by correct responses to experimental stimuli at a rate of 65% or greater.
Figure 1.
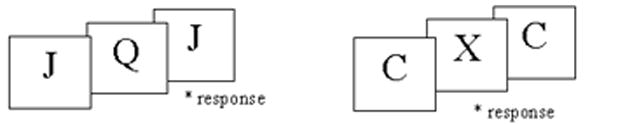
Example of the 2-back task subjects viewed during scanning. (A) Experimental Block- participants pressed the response button when they saw the same letter as 2 letters back. (B) Control Block - participants pressed the response button when they saw the letter “X”.
2.4 Image Acquisition
Stimuli were created with the EPRIME software package (Psychological Software Tool, Inc., Pittsburgh, PA). This software was also connected to the button box with which subjects responded to stimuli, and as such, it also served as the stimulus output device and recorded stimulus responses and reaction times. The stimuli were projected via a magnet-compatible projection system onto a screen positioned at the bore of the magnet.
Data were acquired on a 1.5-T Philips scanner, equipped with a quadrature RF head coil and echo-planar imaging (EPI) capabilities. The subject’s head was immobilized, to reduce head motion artifact, using a vacuum beanbag. Functional scans were acquired using a GRE pulse sequence (TR 2,500 msec, TE 60 msec. FA 90, dynamics=188) over 23 axial slices (4mm, 1mm gap) with a 24 cm FOV and a 64 × 64 matrix (in-plane voxel resolution of 3.75 × 3.75 mm). For accurate anatomical localization, matched T1-weighted high-resolution images were acquired during the scanning session.
2.5 Analysis of Behavioral Data
The hit rate, false alarm rate, reaction time and d – prime were calculated for each subject. The hit rate refers to the percentage of trials in which the subject responded correctly with a button press to a stimulus that was seen two times back. The false alarm rate refers to the percentage of trials in which the subject responded with a button press when no response was needed. D-prime represents a summary score of signal detection, and is derived by calculating the difference between the normalized values of the hit rates and false alarm rates (McFall & Treat, 1999). Our goal was to exclude subjects who responded in a random manner to the task. Extant literature on signal detection theory does not provide an optimal cutoff d-prime score (McFall & Treat, 1999). We considered excluding subjects who achieved a hit rate of < 50% and a false alarm rate of > 50%, thereby receiving a d-prime score of 0 or below. However, we chose a more conservative d-prime cutoff of .88, which required that subjects respond with a hit rate > 66% and a false alarm rate < 33%. In this way, we included subjects who may have responded to the task somewhat poorly, but who did not respond randomly. As noted above, this resulted in the exclusion of 1 control subject, 1 sibling, and 7 subjects with VCFS from our original sample of 43 youth for whom we had 2-back data.
2.6 Imaging Data Analysis
Individual and group brain activity analyses based on BOLD signal changes were carried out using FEAT the FMRIB analysis software package (Smith et al., 2004), www.fmrib.ox.ac.uk/fsl). Pre-statistical processing was applied as following: Head movement was corrected using MCFLIRT (Jenkinson et al., 2002), volumes were smoothed with a Gaussian kernal of FWHM 5mm, intensity normalized, and filtered with a nonlinear high pass temporal filter (50 seconds). Non-brain tissue was removed using BET (Smith, 2002).
Individual subject statistical analyses were carried out using FILM (Woolrich et al., 2001). The working memory (WM) and control conditions were each used as event vectors in the model. A head motion vector (derived from motion correction) was used in the model as a covariate of non-interest to remove and further head motion artifact. Statistical parametric maps, of the contrast WM minus Control, using a voxel by voxel fit to the model were calculated (Z> 2.3 and a cluster significance of P=0.05, corrected) (Forman et al., 1995; Friston et al., 1994; Worsley et al., 1992).
Group activity maps were calculated using FLAME (Beckmann et al., 2003) for the VCFS, sibling and Control groups (as well as for our secondary analyses of the subset of VCFS participants who exhibited dorsal activation). These statistical activation maps were thresholded at Z>2.3 and a cluster significance of P=0.05 were registered into standard MNI space using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Coordinates of local maxima were located in MNI space for identification of activated brain regions.
Group contrasts (ie., Control – VCFS; Siblings – VCFS; VCFS – Controls) were also calculated using FLAME. Contrast masking was employed to remove any influence that negative z values may have had on the resultant activity maps. The statistically significant group activation map (Z>2.3, cluster significance of P=0.05) of the group being subtracted had any voxels with a Z<0 removed from the analysis. Fixed analyses were utilized to take into account within session variances. The statistical activation maps were thresholded with the above-mentioned values and MNI coordinates of clusters and local maxima were noted.
3. Results
3.1 Demographic Characteristics
Study groups did not differ significantly in age (F [df 2,34] = .54 ; p = .59; η2 =.03) or gender ratio (χ2 = .26; p = .88). All study groups differed significantly in full-scale IQ scores (VCFS < controls < siblings) (F [df :2,34] = 31.9; p < .000; η2 =.65). Sixty-five percent of children with VCFS and 60% of controls were diagnosed with a learning disability (χ2 = .06; p = .81), based on the criteria described above. No sibling met our criteria for a diagnosis of a learning disability.
3.2 Behavioral Analysis
Study groups did not differ in reaction time to the experimental condition (F [df 2,34] = 1.35; p = .27; η2 =. 07). D-prime scores did not differ significantly between study groups, although VCFS-affected children tended to perform below the level of their siblings (Overall ANOVA: F [df 2,34] = 2.04; p = .15; η2 =. 11; Sibling/VCFS contrast: p = .06). As noted above, d-prime was calculated on the basis of the hit rate and the false alarm rate. Study groups differed significantly in hit rate (F [df 2,34] = 4.6; p = .02; η2 =.21), due primarily to the differences between VCFS-affected children and their siblings (p = .007); however, the hit rate of children with VCFS tended to be lower than controls as well (p = .07). Study groups did not differ significantly in the rate of false alarms (F [df 2,34] = 1.89; p = .17; η2 =.10).
Although study groups differed significantly in IQ scores, as noted above, within – group correlations between IQ and d-prime scores were not significant for any group (Controls: R = .19; p = .59; Siblings: R = −.05; p = .89; VCFS: R = .20; p = .45).
3.3 Brain Activation: Whole Brain Analysis
3.3.1 Community control participants
Relative to the control condition, community controls exhibited significant activation during the experimental condition in the left precentral gyrus and cingulate, the right inferior frontal gyrus (pars opercularis), and the right superior parietal lobule. (Figure 2) Control participants activated a total of 2,324 voxels in the frontal lobe (spanning Brodmann areas [BA] 6, 32, 44, 45, and 48), and a total of 3,587 voxels in the parietal and occipital lobes, primarily in BA 7, 39, and 40).
Figure 2.

Activation map of the 2-back cognitive task minus control task in control subjects. Axial slices are orientated in radiological convention. R= right brain, L= left brain. Superior- inferior distance from the AC-PC line is indicated. The bar is a visual representation of the range of z values present in this activation map.
3.3.2 Siblings
During the experimental condition (relative to the control condition) siblings showed significant activation in the thalamus, the left lingual gyrus, the left and right middle (dorsolateral prefrontal) and inferior frontal (pars opercularis) gyri, the left and right cingulate, the left and right angular gyrus, the right cuneus and the right occipital lobe. (Figure 3.) Siblings activated a total of 4,598 voxels in the frontal lobe (spanning BA 9, 10, 24, 32, 44, 45, 46, and 48), 4188 voxels in the parietal/occipital lobes (primarily in BA 7, 19 and 40), and 435 voxels in the thalamus.
Figure 3.

Activation map of the 2-back cognitive task minus control task in sibling subjects. See Figure 2 for abbreviations.
3.3.3 VCFS Participants
During the experimental condition (relative to the control condition) individuals with VCFS exhibited significant activation in the left and right inferior frontal gyri and the left and right inferior parietal lobule. (Figure 4.) Participants with VCFS activated a total of 772 voxels in the frontal lobe (primarily in BA 44, 45 and 48) and 3,905 voxels in the parietal and occipital lobes, primarily in BA 7, 19 and 40.
Figure 4.

Activation map of the 2-back cognitive task minus control task in VCFS subjects. See Figure 2 for abbreviations.
3.3.4 Control/VCFS Contrasts
A control – VCFS contrast indicated that relative to VCFS participants, community controls displayed increased activation in the right middle and inferior frontal gyri (BA 45, 46, and 48), the left and right inferior and superior parietal lobules (BA 7, 39 and 40), and the left and right occipital lobes (BA 19). (Table 1, Figure 5A.) A VCFS – control contrast indicated that relative to controls, VCFS participants displayed increased activation in the left orbitofrontal cortex (BA 11), the right cingulate (BA 32), and the right cuneus and occipital cortex (BA 19) (Table 2, Figure 5B).
Table 1.
Location (MNI coordinates) of local maxima voxels for significant clusters for control - VCFS contrast
| Brodmann Area | Brain Region | Z-score | X | Y | Z | Cluster Index/# Voxels |
|---|---|---|---|---|---|---|
| 48 | R Inferior Frontal | 3.51 | 40 | 30 | 18 | 1/319 |
| 46 | R Middle Frontal | 3.49 | 36 | 46 | 20 | 1 |
| 45 | R Middle Frontal | 4.71 | 46 | 40 | 24 | 1 |
| 39 | L Angular | 5.33 | −42 | −52 | 36 | 2/1090 |
| 7 | L Angular | 6.19 | −34 | −64 | 44 | 2 |
| 40 | L Inferior Parietal | 5.59 | −44 | −54 | 48 | 2 |
| 19 | L Middle Occipital | 4.42 | −28 | −66 | 32 | 2 |
| 40 | R Inferior Parietal | 5.77 | 38 | −54 | 44 | 3/1389 |
| 7 | R Angular | 5.39 | 34 | −64 | 44 | 3 |
| 7 | R Superior Parietal | 5.25 | 30 | −70 | 54 | 3 |
| 7 | R Superior Occipital | 5.58 | 32 | −78 | 46 | 3 |
Figure 5.
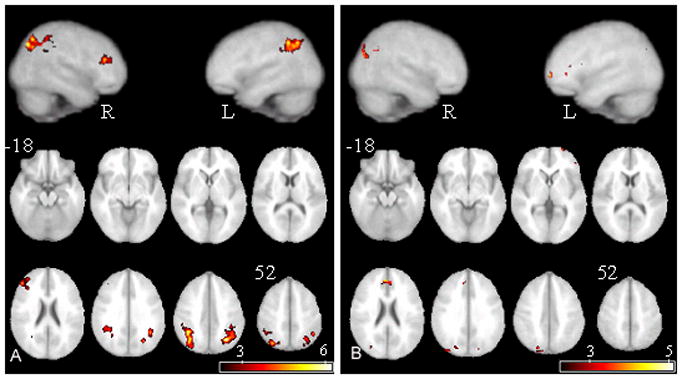
Activity resultant from the subtraction of (A) VCFS subjects’ activation map from control subjects’ activation map (B) control subjects’ activation map from VCFS subjects’ activation map. See Figure 2 for abbreviations.
Table 2.
Location (MNI coordinates) of local maxima voxels for significant clusters for VCFS – control contrast
| Brodmann Area | Brain Region | Z-score | X | Y | Z | Cluster Index/# Voxels |
|---|---|---|---|---|---|---|
| 19 | R Medial Occipital | 3.65 | 44 | −80 | 28 | 1/121 |
| 19 | R Medial Occipital | 3.76 | 36 | −84 | 34 | 1 |
| 19 | R Superior Occipital | 3.51 | 22 | −86 | 38 | 2/145 |
| 19 | R Cuneus | 3.15 | 8 | −86 | 38 | 2 |
| 19 | R Superior Occipital | 3.86 | 20 | −78 | 40 | 2 |
| 11 | L Inferior Frontal | 4.41 | 0 | 44 | 24 | 3/52 |
| 32 | R Anterior Cingulate | 4.02 | 8 | 40 | 28 | 4/194 |
| 32 | R Anterior Cingulate | 4.07 | 6 | 46 | 30 | 4 |
3.3.5 Sibling/VCFS Contrasts
A sibling – VCFS contrast indicated that relative to VCFS participants, siblings displayed increased activation in the left and right cingulate (BA 24 and 32), the left and right angular gyrus (BA 7 and 39), and the left and right occipital lobe (BA 19). (Table 3, Figure 6.) A VCFS – sibling contrast yielded no significant net activation.
Table 3.
Location (MNI coordinates) of local maxima voxels for significant clusters for sibling – VCFS contrast
| Brodmann Area | Brain Region | Z-score | X | Y | Z | Cluster Index/# Voxels |
|---|---|---|---|---|---|---|
| 24 | R Anterior Cingulate | 4.55 | 0 | 28 | 28 | 1/407 |
| 24 | L Middle Cingulate | 4.61 | 0 | 14 | 40 | 1 |
| 32 | L Middle Cingulate | 4.53 | 2 | 14 | 44 | 1 |
| 24 | L Supp Motor Area | 3.54 | −4 | 6 | 48 | 1 |
| 39 | R Angular | 4.32 | 38 | −62 | 44 | 2/536 |
| 7 | R Superior Occipital | 3.67 | 36 | −78 | 46 | 2 |
| 7 | R Angular | 4.26 | 38 | −64 | 48 | 2 |
| 19 | L Middle Occipital | 3.76 | −34 | −78 | 12 | 3/815 |
| 39 | L Angular | 4.3 | −40 | −64 | 46 | 3 |
| 19 | L Middle Occipital | 5.65 | −26 | −74 | 38 | 3 |
| 19 | L Middle Occipital | 4.04 | −30 | −86 | 40 | 3 |
Figure 6.

Activity resultant from the subtraction of VCFS subjects’ activation map from siblings’ activation map
3.4 Analyses of Performance – Matched Groups
Since group differences in performance nearly reached significance, we reanalyzed the data on a subset of subjects who were matched for performance. This subsample consisted of 9 youth with VCFS (6 females), 8 controls (4 females), and the original 10 siblings (5 females). Subgroup differences in hit rate (F [2,24] = 1.76; p = .19; Scheffe post-hoc contrasts: VCFS vs. controls, p = .31; VCFS vs. siblings, p = .27), false alarms (F [2,24] = 1.81; p = .19; Scheffe post-hoc contrasts: VCFS vs. controls, p = .80; VCFS vs. siblings, p = .19), and d-prime scores (F [2,24] = .604; p = .55; Scheffe post-hoc contrasts: VCFS vs. controls, p = .99; VCFS vs. siblings, p = .55) were not significant.
3.4.1 Control/VCFS Contrasts
Several patterns of activation were comparable to the contrasts for the total sample: relative to the VCFS subgroup, the control subgroup displayed increased activation in the right middle and inferior frontal gyri (BA 45, 46, and 48), the left and right inferior and superior parietal lobules (BA 7, 39 and 40), and the left and right occipital lobes (BA 7). However, the control subsample also displayed increased activation in the left inferior frontal operculum (BA 44 and 48), the left precentral gyrus (BA 6) and the cingulum (BA 24 and 32) relative to the performance – matched VCFS subgroup. (See Table 4, Figure 7.)
Table 4.
Location (MNI coordinates) of local maxima voxels for significant clusters for performance-matched control - VCFS contrast
| Brodmann Area | Brain Region | Z score | X | Y | Z | Cluster Index/# voxels |
|---|---|---|---|---|---|---|
| 6 | L Precentral Gyrus | 4.19 | −44 | 0 | 36 | 1/286 |
| 44 | L Inferior Frontal Operculum | 4.12 | −40 | 18 | 36 | 1 |
| 44 | L Inferior Frontal Operculum | 3.27 | −52 | 22 | 34 | 1 |
| 48 | L Inferior Frontal Operculum | 2.95 | −44 | 12 | 24 | 1 |
| 24 | R Middle Cingulum | 5.67 | 4 | 12 | 42 | 2/382 |
| 6 | L Superior Motor Area | 3.54 | −4 | −4 | 58 | 2 |
| 32 | R Middle Cingulum | 3.23 | 6 | 22 | 40 | 2 |
| 44 | R Precentral Gyrus | 4.62 | 52 | 10 | 32 | 3/907 |
| 45 | R Middle Frontal Gyrus | 4.2 | 46 | 38 | 20 | 3 |
| 48 | R Inferior Frontal Operculum | 3.96 | 40 | 6 | 26 | 3 |
| 44 | R Inferior Frontal Operculum | 3.76 | 46 | 10 | 30 | 3 |
| 7 | L Angular Gyrus | 5.5 | −34 | −62 | 44 | 4/1,240 |
| 40 | L Inferior Parietal Lobule | 5.4 | −44 | −54 | 48 | 4 |
| 7 | L Middle Occipital Lobe | 5.34 | −30 | −64 | 40 | 4 |
| 39 | L Angular Gyrus | 5.14 | −40 | −60 | 44 | 4 |
| 40 | L Angular Gyrus | 4.4 | −42 | −48 | 36 | 4 |
| 7 | R Superior Occipital Lobe | 5.9 | 32 | −78 | 46 | 5/1,366 |
| 7 | R Angular Gyrus | 5.42 | 34 | −62 | 44 | 5 |
| 40 | R Inferior Parietal Lobule | 4.77 | 38 | −54 | 44 | 5 |
| 7 | R Superior Parietal Lobule | 4.74 | 32 | −72 | 54 | 5 |
| 40 | R Inferior Parietal Lobule | 4.73 | 34 | −48 | 42 | 5 |
L=Left, R= Right
Minus denotes left, posterior, and ventral to the anterior commisure for x, y, and z coordinates, respectively.
Figure 7.
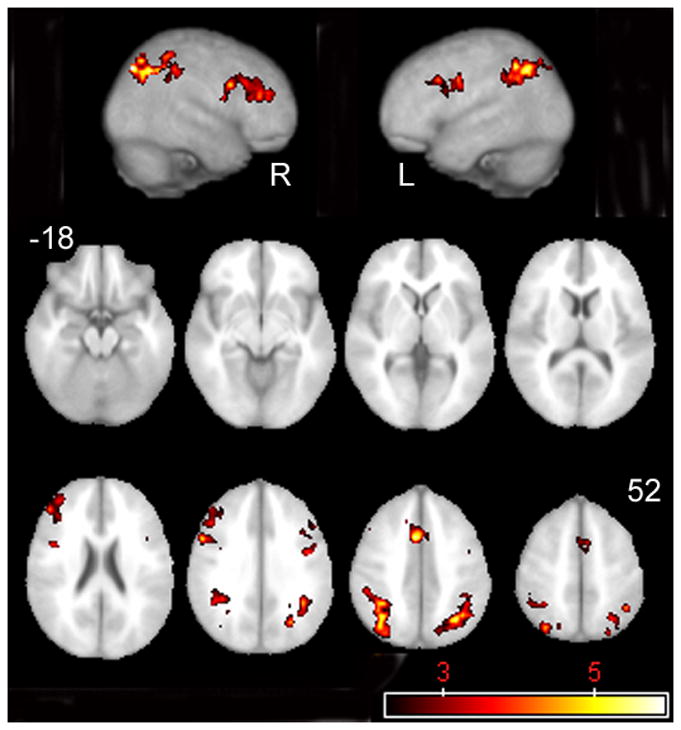
Activity resultant from the subtraction of performance-matched VCFS subjects’ activation map from control subjects’ activation map.
3.4.2 Sibling/VCFS Contrasts
Several patterns of activation were comparable to the contrasts for the total sample: relative to the VCFS subgroup, siblings displayed increased activation in the left and right cingulate (BA 24 and 32), the left and right angular gyrus (BA 7 and 39), and the left and right occipital lobe (BA 19 and 7). In addition, however, siblings displayed increased activation in the left precentral gyrus (BA 6), the left frontal operculum (BA 44), and the left and right thalamus relative to the performance-matched VCFS subgroup. (See Table 5, Figure 8.)
Table 5.
Location (MNI coordinates) of local maxima voxels for significant clusters for performance-matched sibling - VCFS contrast
| Brodmann Area | Region | Z score | X | Y | Z | Cluster Index/# voxels |
|---|---|---|---|---|---|---|
| R Thalamus | 3.04 | 8 | −26 | 8 | 1/270 | |
| L Thalamus | 2.9 | −10 | −22 | 8 | 1 | |
| 6 | L Precentral Gyrus | 3.96 | −48 | 0 | 30 | 2/280 |
| 44 | L Inferior Frontal Operculum | 3.43 | −50 | 12 | 24 | 2 |
| 32 | L Middle Cingulum | 4.52 | 0 | 14 | 40 | 3/606 |
| 24 | L Middle Cingulum | 4.46 | 2 | 14 | 44 | 3 |
| 8 | R Middle Frontal Lobule | 3.44 | 22 | 16 | 50 | 3 |
| 24 | R Anterior Cingulum | 3.37 | 2 | 22 | 30 | 3 |
| 39 | R Angular Gyrus | 4.31 | 38 | −64 | 44 | 4/687 |
| 7 | R Angular Gyrus | 4.3 | 36 | −64 | 48 | 4 |
| 19 | R Middle Occipital Lobe | 3.79 | 38 | −66 | 32 | 4 |
| 7 | R Superior Occipital Lobe | 3.56 | 36 | −78 | 46 | 4 |
| 19 | L Middle Occipital Lobe | 5.38 | −26 | −74 | 38 | 5/1,137 |
| 39 | L Angular Gyrus | 3.78 | −40 | −62 | 28 | 5 |
| 7 | L Angular Gyrus | 3.77 | −38 | −70 | 42 | 5 |
| 18 | L Superior Occipital Lobe | 3.7 | −20 | −72 | 32 | 5 |
L=Left, R= Right
Minus denotes left, posterior, and ventral to the anterior commisure for x, y, and z coordinates, respectively.
Figure 8.

Activity resultant from the subtraction of performance-matched VCFS subjects’ activation map from siblings’ activation map.
4. Discussion
4.1 Overview of findings
To our knowledge, this is the first report of the neural correlates of non-spatial working memory in youth with VCFS. Although the overall d-prime score did not differ between study groups, the hit rate for siblings was significantly higher than for participants with VCFS, who tended to perform less accurately than controls as well. Whole brain analyses indicated that the sibling group recruited a network of 9,221 voxels consisting of DLPFC, Broca’s area, anterior cingulate, superior and inferior parietal lobules, and occipital lobe. Siblings recruited roughly equivalent numbers of voxels in the frontal lobe as the parietal/occipital lobes. Controls recruited a smaller network of 5,911 voxels including Broca’s area, the anterior cingulate, superior and inferior parietal lobules and the occipital lobe. In contrast to siblings, controls recruited somewhat fewer voxels in the frontal lobe, and virtually no significant voxels in the DLPFC. Children with VCFS also recruited a smaller (4,611 voxels) network than siblings; however, in contrast to siblings and controls, significantly activated voxels in the parietal lobe exceeded those in the frontal lobe by a ratio of about 5:1. Activation in the frontal lobe was restricted primarily to Broca’s area in youth with VCFS.
These findings were even more pronounced when we reanalyzed the data on a subset of subjects who were matched for performance. Relative to performance-matched youth with VCFS, both the control and the sibling samples recruited significantly more voxels in the frontal cortex, including the cingulate, the precentral gyrus and to some extent, the operculum.
Accordingly, our data suggest that siblings recruited a prefrontal - parietal network that has been robustly identified in previous studies of working memory in typical children (Casey et al., 1995; Klingberg, 2006; Klingberg et al., 2002; Kwon et al., 2002; Nelson et al., 2000; Thomas et al., 1999) and typical adults (Cabeza et al., 2002); see review by Cabeza and Nyberg (2000). Whereas parietal activation in siblings was roughly equivalent to that of children with VCFS and controls (in both magnitude and location), the frontal component of this network appears to be disrupted in children with VCFS and to a lesser extent in controls. Secondary analyses comparing performance-matched subgroups suggest that frontal lobe disruption is most likely not due to performance differences between groups.
4.2 Frontal lobe disruption in VCFS
This pattern of activation in children with VCFS was unexpected for two reasons. We had anticipated relatively reduced recruitment of the parietal lobe in VCFS participants in light of the anatomic MRI literature that suggests that the volumes of the parietal lobe are significantly smaller in children with VCFS. In addition, reduced activation of the frontal lobe was unexpected insofar as several volumetric studies report that the frontal lobe is preserved in children with VCFS. Our findings suggest that frontal lobe function is disrupted in youth with VCFS despite the relative preservation of frontal lobe volumes.
4.2.1 Absence of dorsolateral prefrontal activation in VCFS and control samples
Both the control and the VCFS groups did not recruit the dorsal prefrontal cortex during this task. Previous imaging studies of working memory suggest that the ventral prefrontal cortex supports the maintenance component of working memory and that the dorsolateral prefrontal cortex (DLPFC) supports the update/executive component of working memory (Fletcher & Henson, 2001; Collette & Van der Linden, 2002). Since extant neuropsychological studies of working memory in either VCFS or idiopathic learning disabilities do not dissociate the maintenance and update components of the task, we do not know specifically which aspects of working memory poses the greatest challenge for youth with VCFS or controls. However the absence of DLPFC activation supports the notion that the update component of working memory may have been challenging for both VCFS youth and community controls. The activation patterns of both controls and VCFS participants suggested that instead of recruiting the dorsolateral prefrontal cortex (DLPFC) when they attempt to update information, children in both study groups compensated by relying primarily on phonological rehearsal, as suggested by their relatively robust recruitment of the operculum (although results from the performance – matched samples suggest that the controls recruit more voxels in the operculum than youth with VCFS). The presence of learning disabilities in both the control and the VCFS samples suggests that the neurocognitive dysfunction that is common to both samples may have contributed to the absence of DLPFC activation.
4.2.2. Absence of cingulate activation in VCFS sample, but not control sample
Our data suggest that reduction in frontal lobe activation extends beyond the DLPFC in children with VCFS. In contrast to both controls and siblings, children with VCFS did not recruit the cingulate. The cingulate is thought to play a role in the executive component of working memory, through its mediation of cognitive control and allocation of attention (Otsuka et al., 2006; Bush et al., 2000). Bish and colleagues (2005) have demonstrated pronounced deficits in executive control in children with this disorder on tasks requiring conflict monitoring, thus supporting the notion that a neural network that includes the DLPFC and anterior cingulate is disrupted. Interestingly, however, Scherf and colleagues (2006) have noted developmental maturation in the neural circuit underlying working memory functions. They found that whereas children exhibited limited activation in DLPFC and virtually no activation in cingulate during a spatial working memory task, adolescents relied much more heavily on these regions. These findings raise the question of whether the absence of DLPFC and cingulate activation in VCFS is due to a delay, as opposed to a disruption, in the prefrontal aspect (ie., DLPFC and cingulate) of the circuit that subserves working memory. Either longitudinal studies, or cross-sectional studies that include larger samples spanning a wider age range, would help to clarify this question.
Neural “hypoactivation” is often attributable to performance deficits on functional tasks. Since children with VCFS tended to perform more poorly than their peers, we analyzed a subset of performance-matched subjects in order to rule out the contribution of performance deficits to the relative absence of frontal lobe activation in youth with VCFS. We found that relative to controls and siblings, performance – matched subjects with VCFS continued to demonstrate less frontal lobe activation; instead, they relied primarily on posterior brain regions when completing the task. However, we cannot rule out the possibility that other behavioral factors could have contributed to prefrontal “hypoactivation” as well, including increased distractibility, inability to keep pace with the rapid rate of stimulus presentation, or lack of motivation (Manoach, 2003).
In sum, these data suggest that the absence of expected activation in the DLPFC (BA 10 and 46) in the control and VCFS samples may be due to the neurocognitive dysfunction that is present in both study groups. However, the frontal hypoactivation that we observed in children with VCFS extended beyond DLPFC disruption to the cingulate, suggesting that children with VCFS may have difficulty bringing to bear their neural resources on several components of working memory, including not only update functions but also cognitive control and attentional allocation. This is consistent with previous reports of prefrontal (Kates et al., 2004) alterations, as well as of deficits in the frontal-mediated executive control system in children with this disorder.
4.3 Limitations
Our data are limited to VCFS-affected children whose working memory skills were relatively intact. Thirty percent of the total number of VCFS-affected children to whom we administered the task obtained a d-prime score of lower than .88, and therefore were excluded from analyses. Although the demographic characteristics of children who were excluded did not differ from those who were included, our results may not be generalizable to children with VCFS who have more severe working memory deficits. Nonetheless, we believe that the inclusion of poor performers in the sample would have posed challenges to data interpretation.
We cannot rule out the possibility that group differences in IQ scores contributed to the differences in behavioral performance or patterns of activation during the working memory task. However, we found that within-group correlations between IQ and behavioral performance were not significant. Although the absence of significant correlations may be due to lack of power, it also may suggest that IQ does not affect performance on working memory tasks in intellectually-disabled samples to extent that it does in more typical populations. Future analyses with larger, IQ-stratified samples could clarify this question.
4.4 Implications
Several volumetric imaging reports, including our own, have reported that frontal lobe is relatively preserved, whereas posterior regions, particularly the parietal lobe, are affected in VCFS. The data that we report here suggest that despite the relative preservation of prefrontal cortex volume, its function appears to be disturbed. This is consistent with neuropsychological studies that demonstrate that children with VCFS have deficits in frontal-based skills such as executive function, cognitive control and allocation of attention (Bearden et al., 2001; Sobin et al., 2004; Van Amelsvoort et al., 2004; Bish et al., 2005). Future research should consider how best to apply the apparent reliance of VCFS-affected children (and potentially, children with idiopathic learning disabilities) on Broca’s area during working memory tasks to the educational domain. It seems plausible that phonological rehearsal/repetition may be effective in helping these children maintain non-spatial information.
Acknowledgments
This study was supported by grants from the National Institutes of Health (MH 64824 and MH 65481) and from a Hendricks Grant from the State University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baddeley A. Working Memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D. COMT Val108/158Met Modifies Mismatch Negativity and Cognitive Function in 22q11 Deletion Syndrome. Biol Psychiatry. 2005:1–9. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Emannuel B, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23:447–64. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General Multi-level linear modelling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bish JP, Ferrante SM, McDonald-McGinn D, Zackai E, Simon TJ. Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11.2 deletion syndrome. Developmental Science. 2005;8:36–43. doi: 10.1111/j.1467-7687.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. Parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in the anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nybergs L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kayson D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2:221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neuroscience and Biobehavioral Reviews. 2001;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Dowker A. What can functional brain imaging studies tell us about typical and atypical cognitive development in children? J Physiology, Paris. 2006;99:333–341. doi: 10.1016/j.jphysparis.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Driscoll DA, Budarf ML, Emanuel BS. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. Am J Hum Genet. 1992;50:924–933. [PMC free article] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: A volumetric MRI study. Am J Psych. 2000;157:409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: Usefulness as phenotypic indicators of schizophrenia risk. Biol Psychiatry. 2002;51:312–318. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Barnes M, Stuebing KK, Francis DJ, Olson R, et al. Classification of learning disabilities: An evidence-based evaluation. In: Bradley R, Danielson L, Hallahan DP, editors. Identification of learning disabilities: Research to practice. Erlbaum; Mahwah, N.J: 2002. pp. 185–250. [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KL, Worsley KJ, Fralkowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activation using their spatial extent. Human Brain Mapping. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskyn M, Swanson HL. Cognitive processing of low achievers and children with reading disabilities: A selective meta-analytic review of the published literature. The School Psychology Review. 2000;29:102–119. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kates W, Burnette C, Bessette B, Folley B, Strunge L, Jabs E, Pearlson G. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) J Child Neurol. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- Kates W, Burnette C, Jabs E, Rutberg J, Murphy A, Grados M, Geraghty M, Kaufmann W, Pearlson G. Regional cortical white matter reductions in velocardiofacial syndrome: a volumetric MRI analysis. Biol Psychiatry. 2001;49:677–685. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AMC, Naidu S, Kaufmann WE, Reiss AL. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Research: Neuroimaging. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children present and lifetimeversion (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2005.11.019. in press. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci USA. 2002;99:13336–41. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajiness-O’Neill RR, Beaulieu I, Titus JB, Asamoah A, Bigler ED, Bawle EV, Pollack R. Memory and learning in children with 22q11.2 deletion syndrome: evidence for ventral and dorsal stream disruption? Neuropsychol Dev Cogn C Child Neuropsychol. 2005;11:55–71. doi: 10.1080/09297040590911202. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. European Journal of Neuroscience. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- McFall RM, Treat TA. Quantifying the information value of clinical assessments with signal detection theory. Annu Rev Psychol. 1999;50:215–241. doi: 10.1146/annurev.psych.50.1.215. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The ontogeny of human memory: A cognitive neuroscience perspective. Developmental Psychology. 1995;31:723–738. [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36:109–16. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Osaka N, Morishita M, Kondo H, Osaka M. Decreased activation of anterior cingulate cortex in the working memory of the elderly. Neuroreport. 2006;17:1479–1482. doi: 10.1097/01.wnr.0000236852.63092.9f. [DOI] [PubMed] [Google Scholar]
- Robin N, Shprintzen R. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147:90–96. doi: 10.1016/j.jpeds.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Scambler PJ, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R, Wilson DI, Goodship JA, Cross IE, Burn J. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- Scherf SK, Sweeney JA, Luna B. Brain Basis of Developmental Change in Visuospatial Working Memory. Journal of Cognitive Neuroscience. 2006;7:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Shprintzen R. Velo-cardio-facial syndrome. Prog Pediart Cardiol. 2005;20:187–193. [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. NeuroImage. 2005;25:169–80. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust Automated Brain Extraction. Human Brain Mapping. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, DeLuca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, DeStefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M. Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol. 2004;26:611–626. doi: 10.1207/s15326942dn2602_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, Blundell M, Anyane-Yeboa K, Karayiorgou M. Neuropsychological characteristics of children with the 22q11 Deletion Syndrome: a descriptive analysis. Neuropsychol Dev Cogn C Child Neuropsychol. 2005;11:39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE, Siegel LS. Phenotypic performance profiles of children with reading disabilities: A regression based test of the phonological-core variable difference model. Journal of Educational Psychology. 1994;86:24–53. [Google Scholar]
- Stuebing KK, Fletcher JM, LeDoux JM, Lyon GR, Shaywitz SE, Shaywitz BA. Validity of IQ discrepancy classifications of reading disabilities: A meta-analysis. American Educational Research Journal. 2002;39:469–518. [Google Scholar]
- Swillen A, Devriendt K, Ghesquiere P, Fryns J. Children with a 22q11 deletion versus children with a speech-language impairment and learning disability: behavior during primary school age. Genet Couns. 2001;12:309–317. [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J Med Genet. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10:327–38. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, Critchley H, Owen MJ, Henry J, Murphy KC, Murphy DGM. Structural brain abnormalities associated with deletion at chromosome 22q11: Quantitative neuroimagiang study of adults with velo-cardio-facial syndrome. British Journal of Psychiatry. 2001;178:412–419. doi: 10.1192/bjp.178.5.412. [DOI] [PubMed] [Google Scholar]
- Van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophr Res. 2004;70:223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test - II. San Antonio: 2001. [Google Scholar]
- Woolrich WM, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]


