Abstract
HIV-1 Nef protein shares a significant homology with the immunosuppressive and highly conserved retroviral transmembrane protein p15E. In the present study, extracellular Nef protein is shown to induce interleukin (IL)-10 mRNA expression in human peripheral blood mononuclear cells as well as in cells of H9 T and U937 promonocytic human cell lines. Release of IL-10 protein into supernatants of peripheral blood mononuclear cells stimulated with Nef is dose-dependent. Expression of cytokines IL-2, IL-4, IL-5, IL-12 p40, IL-13, and interferon γ is not affected by Nef stimulation. IL-10 protein production induced by Nef is inhibited by the calcium/calmodulin phosphodiesterase inhibitor W-7 but not by the protein kinase A inhibitor H-89 nor the protein kinase C inhibitors staurosporine and calphostin C. The calcium chelating agent EGTA also inhibits the IL-10 production induced by Nef, and this inhibition is reversed by the addition of calcium along with Nef. These findings indicate that extracellular Nef may contribute to the immunopathogenesis of HIV infection by inducing IL-10.
Nef, a highly conserved 25- to 30-kDa regulatory protein that is produced early in the HIV-1 lentivirus life cycle, is gaining increasing importance in the analysis of pathogenesis of HIV infection (1). Nef is critical to the maintenance of a high viral load and for the development of AIDS (2, 3). Although the complete function of Nef remains enigmatic, Nef has been shown to enhance viral infectivity and replication in primary cells (4, 5), induce down-regulation of CD4 (6, 7) and major histocompatibility complex class I surface expression (8), and alter the activation of T cells (3, 9, 10). Deletions and mutations in the nef gene have been associated with long-term survival in patients infected with HIV (11, 12). Further, simian immunodeficiency virus-infected macaques initially infected with nef(−) simian immunodeficiency virus are protected from subsequent infection by pathogenic nef(+) simian immunodeficiency virus (13).
The predominantly cytoplasmic, myristylated Nef protein is highly immunogenic (14, 15). Nef-specific antibodies and cytotoxic T lymphocytes are present in the circulation of two-thirds of HIV-seropositive individuals (16–18) indicating that Nef may be present extracellularly in vivo. In vitro, studies show that HIV-infected cells release Nef protein in vesicles as observed by confocal laser-scanning microscopy and by its sedimentation behavior on ultracentrifugation (19, 20). Further, HIV-1 Nef protein derived from yeast cells has been found in the extracellular medium in concentrations of up to 40 μg/ml during stress (21). The amino-terminal region of the 27-kDa Nef protein has membrane fusion properties that may be involved in its extracellular release (22).
Recently the analysis of extracellular Nef using CD4+ T cell lines has shown that the carboxyl-terminal region of Nef is located extracellularly (23). This surface domain of Nef appears to play an important role in the interaction of Nef-infected cells with CD4+ uninfected cells and contributes to HIV-induced syncytium formation. Extracellular Nef has also been observed to induce activation of HIV infection in latently infected MOLT-20-2 cells (24). Other studies have shown that extracellular Nef inhibits the proliferation of CD4+ T cells and decreases the response of HIV-uninfected peripheral blood mononuclear cells (PBMCs) and purified T cells to the mitogen phytohemagglutinin (25).
Cytokine dysregulation contributes to the abnormalities of immune function in HIV infection that appear even before the development of profound CD4+ lymphopenia and disease progression (26, 27). HIV-infected individuals have a progressive loss of type 1 cellular immune responses, mediated by interleukin (IL)-2, interferon γ (IFN-γ), and IL-12, and develop predominantly type 2 humoral immune responses, mediated by IL-4, IL-5, IL-6, and IL-10 (28, 29).
In vitro studies show that immunosuppressive retroviral components, such as the highly conserved transmembrane envelope protein p15E of several animal and human retroviruses or its synthetic homologous peptide, CKS-17, may contribute to cytokine dysregulation (30). Since Nef proteins display amino acid sequence homology to p15E (31), it seemed logical to examine the influence of extracellular HIV-1 Nef protein on type 1 and type 2 cytokine expression. Our results show that Nef protein induces expression of IL-10 in both human PBMCs and the two human cell lines tested and that induction of IL-10 by extracellular Nef involves the calcium/calmodulin signal transduction pathway.
MATERIALS AND METHODS
Nef Protein.
HIV-1 LAV Nef protein was obtained through the AIDS Research and Reference Reagent Program, AIDS Program, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; Rockville, MD). Nef, a 25.7-kDa recombinant protein, is produced by the Escherichia coli strain Sφ930 transfected with the HIV-1 nef gene, which was isolated from the bacteriophage pBENN 6 and cloned into the bacterial expression vector pPD-YN-61 (32). The protein was purified by ammonium sulfate precipitation, anion exchange chromatography, ultrafiltration, and cation exchange chromatography and has a purity of >90%. The protein was tested and found free of bacterial endotoxin using the Limulus amebocyte lysate assay (Associates of Cape Cod). A 206-aa myristylated HIV-1 Nef protein produced in the yeast Saccharomyces cerevisiae (21) was a kind gift from Ahmed A. Azad (Biomolecular Research Institute, Victoria, Australia).
Cells.
PBMCs from healthy HIV-negative donors were isolated by density gradient centrifugation using Lymphoprep (Accurate Scientific, Westbury, NY). The human T lymphoid cell line, H9, was obtained from Robert Gallo (33) through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. U937 cells. The human promonocytic cell line (34), was obtained from American Type Culture Collection.
Reverse Transcription–PCR (RT-PCR) of PBMCs, H9, and U937 Treated Cells.
To a 24 h rested culture of PBMCs (1 × 107 cells per ml per well, 12-well plates) in RPMI 1640 medium (GIBCO/BRL) supplemented with 10% heat-inactivated fetal calf serum and 1% penicillin/streptomycin, E. coli-derived Nef (50 ng/ml), yeast-derived Nef (100 ng/ml), staphylococcal enterotoxin A (100 ng/ml; Toxin Technology, Sarasota, FL), or BSA (1 μg/ml) was added. The mixtures were incubated at 37°C for 3, 8, and 24 h in a humidified 5% CO2/95% air incubator.
H9 cells (5 × 106 cells in 5 ml per well, 6-well plates) were cultured for 2, 6, and 18 h in the presence of E. coli-derived Nef (50 ng/ml). Phorbol myristate acetate (10 ng/ml) with ionomycin (1 μg/ml) added to H9 cells was used as control. U937 cells (0.5 × 106 cells per ml) were pretreated for 24 h with 1.2% dimethyl sulfoxide (35). The treated cells were then washed twice with phosphate buffer solution, resuspended in culture medium, and cultured at 3 × 106 cells in 3 ml per well (12-well plates) in the presence of Nef (100 ng/ml) or phorbol myristate acetate (50 ng/ml) for 3, 6, and 18 h at 37°C as described above.
Total cellular RNA was extracted with guanidinium thiocyanate (RNAzol; Tel-Test, Friendswood, TX) according to the protocol of the supplier. cDNA was made from RNA samples as described previously (30). PCR primers were synthesized using an Applied Biosystems 392 DNA/RNA synthesizer. Primer sequences for IL-2, IL-4, IL-5, IL-12 p40 heavy chain, IL-13, and β-actin are shown in Table 1. Primers for IL-10 and IFN-γ were obtained from CLONTECH. The size of the PCR products for IL-10 and IFN-γ were 328 bp and 455 bp, respectively. PCR was initiated in the thermal cycler programmed for 95°C for 15 s, 56°C for 15 s, and 72°C for 75 s for 30 cycles. PCR products were analyzed by electrophoresis, and the gel was photographed as described previously (30). The negative was scanned with the Multiscan-R (Interactive Technologies International, St. Petersburg, FL), and cytokine mRNA levels were normalized to β-actin mRNA levels.
Table 1.
Description of primers used in this study
| Cytokine | Primer sequence | PCR product size, bp |
|---|---|---|
| IL-2-5′ | 5′-AACAGTGCACCTACTTCAAG-3′ | 397 |
| IL-2-3′ | 5′-GTTGAGATGATGCTTTGACA-3′ | |
| IL-4-5′ | 5′-TCTCACCTCCCAACTGCTTCC-3′ | 320 |
| IL-4-3′ | 5′-CGTTTCAGGAATCGGATCAGC-3′ | |
| IL-5-5′ | 5′-TGCCTACGTGTATGCCATCCC-3′ | 437 |
| IL-5-3′ | 5′-CTTGGCCCTCATTCTCACTGC-3′ | |
| IL-12 (P40)-5′ | 5′-TCAAAGAGTTTGGAGATGCTGGCC-3′ | 464 |
| IL-12 (P40)-3′ | 5′-TGATGATGTCCCTGATGAAGAAGC-3′ | |
| IL-13-5′ | 5′-CAGAGGATGCTGAGCGGATTC-3′ | 836 |
| IL-13-3′ | 5′-TGCCTGTGTGTGAAGTGGGTC-3′ | |
| β-actin-5′ | 5′-GTGATGGTGGGCATGGGTCA-3′ | 510 |
| β-actin-3′ | 5′-TTAATGTCACGCACGATTTCCC-3′ |
Northern Blot Analysis.
Northern blot analyses were performed as described previously (30). Human IL-10 (759-bp BglII/HindIII fragment of pH5C) and β-actin (1.1-kb EcoRI fragment of HHC189) probes were used (30). The cDNA plasmids, pH5C and HHC189, were obtained from American Type Culture Collection. Hybridization probes were labeled using a random-primed DNA-labeling kit (Boehringer Mannheim) and [α-32P]dCTP [3000 Ci/mmol (1 Ci = 37 GBq; Dupont/NEN). Autoradiographic films for Northern blot were analyzed by the Multiscan-R.
ELISA for IL-10.
PBMCs were cultured at 4 × 105 cells in 200 μl per well (96-well plates) with E. coli- or yeast-derived Nef at doses of 0, 10, 100, 500, and 1000 ng/ml. Cell viability as tested by trypan blue dye exclusion was always >95%. The supernatants were harvested after 24, 48, and 72 h, centrifuged to remove cell debris, and stored at −70°C until they were analyzed for IL-10 protein levels. IL-10 protein was measured by ELISA (IL-10 Cytoscreen Immunoassay Kit; BioSource International, Camarillo, CA). The minimum detectable dose was ≥5 pg/ml.
Signal Transduction Experiments.
PBMCs (4 × 105 cells in 200 μl per well, 96-well plates), previously rested for 24 h, were separately pretreated for 2 h with signal transduction inhibitors from Calbiochem. These were W-7 (50 μM; calmodulin antagonist IC50 = 28 μM), EGTA (1 mM), H-89 (100 nM; protein kinase A inhibitor Ki = 48 nM), staurosporine [1.5 nM; protein kinase C (PKC) inhibitor IC50 = 0.7 nM], or calphostin C (100 nM; PKC inhibitor IC50 = 50 nM). These mixtures were next stimulated with yeast-derived Nef (500 ng/ml) alone or with 1.4 mM calcium chloride. Supernatants were harvested as described above after 40 h of incubation and tested for IL-10 protein by ELISA.
RESULTS
Nef Protein Induces IL-10 mRNA Expression in PBMCs.
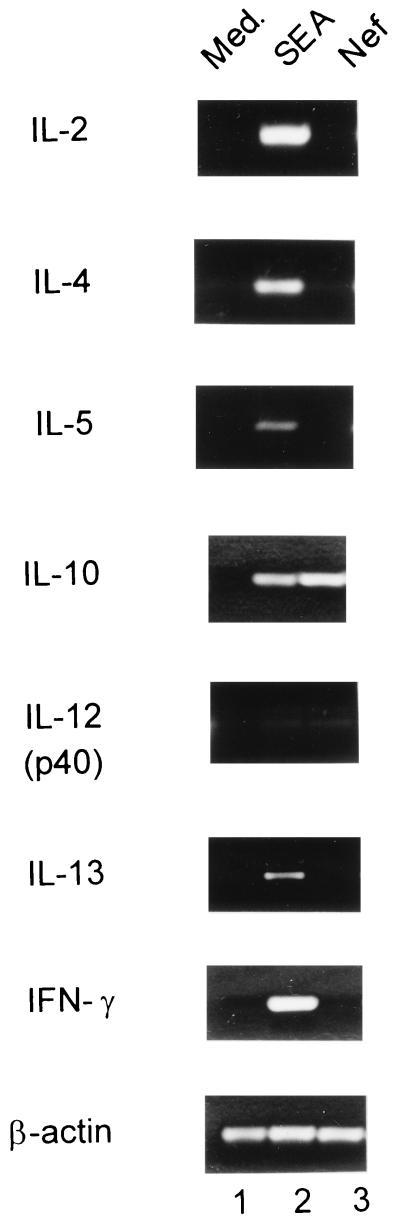
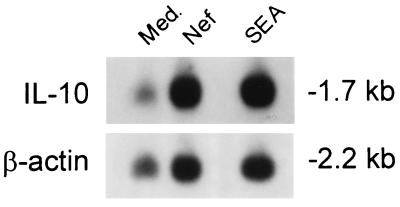
Fig. 1 shows that the expression of IL-10 as revealed by RT-PCR is induced in PBMCs treated for 3 h with Nef derived from E. coli. This was the consistent result of three separate experiments. Other cytokines investigated in an identical manner, IL-2, IL-4, IL-5, IL-12 (p40), IL-13, or IFN-γ, are not so induced. Recombinant Nef protein derived from yeast also induced IL-10 mRNA expression after 3 h of stimulation (data not shown). In contrast, the negative control protein BSA shows no IL-10 induction (data not shown). Fig. 1 also shows that onset of IL-10 mRNA induction is detected as early as 3 h after stimulation with Nef. In other experiments (not shown in Fig. 1), the expression of IL-10 is also demonstrable at 6 and 24 h. In Fig. 2, Northern blot analysis of PBMCs incubated for 24 h with Nef shows a 3-fold increase in IL-10 mRNA expression by densitometric analysis.
Figure 1.
RT-PCR analysis of the effects of E. coli-derived Nef on cytokine mRNA accumulation using human PBMCs. Human PBMCs were incubated for 3 h with medium (lane 1), 100 ng/ml staphylococcal enterotoxin A alone (lane 2), or 50 ng/ml Nef alone (lane 3).
Figure 2.
Northern blot analysis of the effect of of E. coli-derived Nef on IL-10 mRNA expression using human PBMCs. PBMCs were incubated for 24 h with medium, 50 ng/ml Nef alone, or with 100 ng/ml staphylococcal enterotoxin A alone (positive control). Densitometric analysis was performed by reading the autoradiographic negative film with the Multiscan-R. IL-10 mRNA levels were normalized to β-actin mRNA levels. Arbitrary optical density units (AODU) were: medium = 0.45, Nef = 1.33, and staphylococcal enterotoxin A = 1.33.
Induction of IL-10 Protein Production by Nef Using PBMCs Is Dose-Dependent.
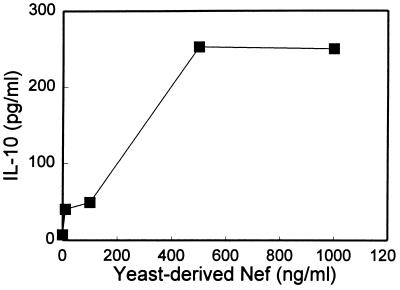
Fig. 3 shows that the production of IL-10 protein using PBMCs cultured at 48 h with varying concentrations of yeast-derived Nef is dose-dependent. As shown, the peak production of IL-10 by yeast-derived Nef is at 500 ng/ml (18.5 nM). Similar dose-dependent responses are obtained with E. coli-derived Nef (data not shown).
Figure 3.
Protein production of IL-10 in supernatants of PBMCs stimulated with increasing doses of yeast-derived Nef for 48 h. ELISA values represented are for pooled culture supernatants from five wells. IL-10 protein levels for those stimulated with 0, 10 (0.37 nM), 100, 500, and 1000 ng/ml of yeast-derived Nef are 7, 40, 49, 253, and 251 pg/ml, respectively. Similar dose-dependent influences of Nef derived from E. coli are also observed.
Nef Protein Induces IL-10 mRNA Expression in H9 or U937 Cells.
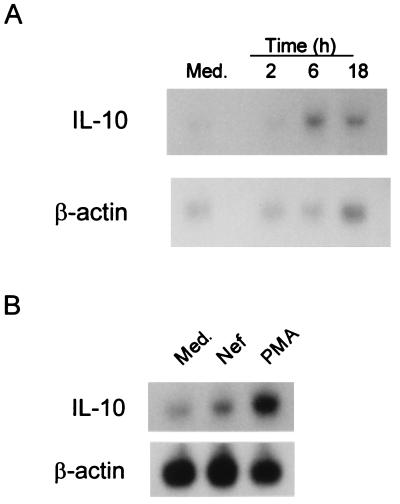
H9 T cells and U937 promonocytic cells both produce IL-10 following HIV infection (36). Consequently, these two cell lines were chosen to study the induction of IL-10 by Nef. Fig. 4A shows by Northern blot analysis using H9 T cells that maximal production of IL-10 mRNA (6-fold over control) was observed 6 h following stimulation with Nef. With U937 cells pretreated with dimethyl sulfoxide and stimulated with Nef (see Materials and Methods), Northern blot analysis also revealed a 2-fold increase of IL-10 mRNA (3 h of stimulation) by densitometric analysis (Fig. 4B).
Figure 4.
Effects of E. coli-derived Nef on IL-10 mRNA expression in H9 and U937 cells. (A) Northern blot analysis of the effect of E. coli-derived Nef on IL-10 mRNA expression in H9 cells after 2, 6, and 18 h of incubation. IL-10 mRNA expression with medium at 6 h of incubation is shown in the left lane. Densitometric analysis reveals the following arbitrary optical density units (AODU): 6 h medium = 0.547, 2 h Nef = 0.534, 6 h Nef = 3.303, and 18 h Nef = 0.881. (B) Northern blot analysis of the effect of E. coli-derived Nef on IL-10 mRNA expression using U937 cells. U937 cells pretreated with 1.2% dimethyl sulfoxide for 24 h were incubated with medium, 100 ng/ml Nef, or 50 ng/ml phorbol myristate acetate (positive control) for 3 h. AODU were: medium = 0.199, Nef = 0.399, and phorbol myristate acetate = 0.784.
IL-10 Protein Production Induced by Nef in PBMCs Involves the Calcium/Calmodulin Phosphodiesterase Signal Transduction.
To evaluate the signal transduction pathway involved in IL-10 production by Nef, PBMCs were exposed to several signal transduction inhibitors in two separate experiments. As shown in Fig. 5, W-7, an inhibitor of calcium/calmodulin-dependent phosphodiesterase, consistently inhibited IL-10 protein production by Nef. Both staurosporine, a PKC inhibitor, and H-89, a protein kinase A inhibitor, has no effect on Nef-induced IL-10 production. Calphostin C, another PKC inhibitor-like staurosporine, also has no affect on IL-10 protein production by Nef. The calcium chelating agent EGTA also shows inhibition of IL-10 protein production by Nef. This inhibition is reversed by the addition of calcium.
Figure 5.
Signal transduction experiments of IL-10 induction by Nef using several inhibitors. PBMCs were pretreated separately for 2 h with medium or inhibitor alone or were additionally stimulated with 500 ng/ml yeast-derived Nef alone or with 1.4 mM calcium chloride for 40 h. IL-10 production in the supernatants of PBMC from two different individuals was detected by ELISA. In Exp. 1, inhibitors used were 50 μM W-7, 1.5 nM staurosporine, and 100 nM H-89. In Exp. 2, inhibitors used were 50 μM W-7, 1.5 nM staurosporine, 100 nM H-89, 100 nM calphostin C, and 1 mM EGTA.
DISCUSSION
In the present study, we demonstrate that HIV-1 Nef protein increases IL-10 mRNA expression, as detected by RT-PCR or Northern blot analysis using PBMCs or cells obtained from a T cell line (H9) or promonocytic cell line (U937). Nef proteins derived from either E. coli or the yeast S. cerevisiae induce the expression of IL-10. Kinetic studies show that IL-10 mRNA induction occurs as early as 3 h (earliest time examined) following stimulation with Nef. The increased mRNA expression parallels production of the protein measured in the PBMC supernatants by ELISA. We further show that induction of IL-10 by extracellular Nef involves the calcium/calmodulin phosphodiesterase pathway.
IL-10, a cytokine produced by monocytes/macrophages, B lymphocytes, and mainly T helper type 2 (Th2) lymphocytes, is a powerful suppressor of cell-mediated immunity. It has pleiotropic effects on various cell lineages, such as suppression of macrophage activity and inhibition of production of IL-1, IL-6, tumor necrosis factor-α, granulocyte–macrophage colony-stimulating factor, and granulocyte colony-stimulating factor. IL-10 also indirectly inhibits T cell proliferation by down-regulating class II major histocompatibility complex antigen expression on the surface of monocytes (37).
HIV-1 Nef is of particular interest to our laboratory, since it shares a significant homology with p15E (31), a conserved transmembrane envelope protein of numerous animal and human retroviruses (38). A synthetic peptide homologous to a conserved domain of p15E, CKS-17, has been shown to be highly immunosuppressive in vitro and in vivo (39–41). CKS-17 down-regulates tumor necrosis factor-α and IL-12 and enhances IL-10 in vitro, suggesting that development of opportunistic infections and/or progression of retrovirus-induced immunodeficiencies may not require intact replicating virus (30, 42).
Few studies describing the effect of Nef on cytokine modulation in human PBMCs have been reported. Chirmule et al. (43) showed that extracellular Nef induces IL-6 at both mRNA expression and protein level using human PBMCs. In these studies, Nef protein was able to induce B cell differentiation into immunoglobulin-secreting cells, and these authors suggested that IL-6 plays an important role in this process.
Cytokine modulation by intracellular Nef protein has been reported using a Jurkat T cell line but not primary lymphocytes (44). In the Jurkat T cell line transfected with the nef gene, IL-2 and IFN-γ were decreased by intracellular Nef protein, but IL-6 and IL-10 were not detected by RT-PCR. Intracellular Nef may not induce IL-6 and IL-10, or Jurkat cells may be an unsuitable cell line to examine the induction of IL-6 and IL-10 by Nef. Alternatively, the extracellular action of Nef may be distinct from its intracellular role.
Our results show that IL-10 induction by extracellular Nef involves the calcium/calmodulin signal transduction pathway and not the protein kinase A or PKC pathway. The signal transduction experiments in the present study suggest that extracellular Nef may bind to a surface receptor or to the calcium channels, leading to activation of the calcium/calmodulin phosphodiesterase pathway. In this context, it has been shown that HIV-1 Nef protein interacts with potassium channels (45), an electrophysiological activity requiring the extracellular presence of Nef and which may be related to Nef signaling. Intracellular Nef has been shown to associate with signaling cascades such as the Src tyrosine kinase family Src homology domains (46, 47) and a serine/threonine kinase that can be inhibited by staurosporine (48).
Extracellular Nef may bind to a membrane receptor or may be taken up by cells as demonstrated with Tat, another regulatory protein (49). Torres et al. (50) have reported binding of extracellular Nef to major histocompatibility complex class II molecules. They proposed that activation of mononuclear cells induced by extracellular Nef may serve as an endogenous mitogen in the infected individual, leading to increased viral replication.
From the virological perspective, IL-10 predominantly inhibits HIV-1 replication in monocytes/macrophages (36, 51, 52). In infectious states caused by organisms such as Epstein–Barr virus or certain parasites, IL-10 may provide virus-infected cells a means of escape from immune surveillance and thus aid in the development of a chronic carrier state (53, 54). In this context, the induction of IL-10 by Nef protein may confer survival advantage for the HIV by inhibiting immediate death of its host cell and aiding in the establishment of viral latency.
PBMCs and lymph nodes of patients infected with HIV spontaneously express IL-10 (36, 51, 55). Further, H9 or U937 cell lines infected with HIV-1 have also been shown to produce a higher expression of IL-10 mRNA (36). Nef protein is produced early in HIV infection (1) and may participate in the cytokine dysregulation observed during HIV infection.
The present results are consistent with these in vivo and in vitro observations, indicating that extracellular Nef might play an important role in the immunopathogenesis and viral infectivity of HIV infection by inducing immunosuppression through induction of IL-10.
Acknowledgments
We are grateful to Claudine Baird for preparation of this manuscript. This work was supported by the Eleanor Naylor Dana Charitable Trust, Ronald McDonald Children’s Charities, and The Newland Foundation.
ABBREVIATIONS
- PBMC
peripheral blood mononuclear cell
- IFN-γ
interferon γ
- IL
interleukin
- PKC
protein kinase C
- RT-PCR
reverse transcription–PCR
References
- 1.Cullen B R. Virology. 1994;205:1–6. doi: 10.1006/viro.1994.1613. [DOI] [PubMed] [Google Scholar]
- 2.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 3.Trono D. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 4.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia J V, Miller A D. Nature (London) 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 7.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard J-M. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 9.Skowronski J, Parks D, Mariani R. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 12.Deacon N J, Tyskin A, Solomon A, Smith K, Ludford-Menting M, et al. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 13.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 14.Bahraoui E, Yagello M, Billaud J-N, Sabatier J-M, Guy B, Muchmore E, Girard M, Gluckman J-C. AIDS Res Hum Retroviruses. 1990;6:1087–1098. doi: 10.1089/aid.1990.6.1087. [DOI] [PubMed] [Google Scholar]
- 15.Kaminchik J, Margalit R, Yaish S, Drummer H, Amit B, Sarver N, Gorecki M, Panet A. AIDS Res Hum Retroviruses. 1994;10:1003–1010. doi: 10.1089/aid.1994.10.1003. [DOI] [PubMed] [Google Scholar]
- 16.Ameisen J-C, Guy B, Chamaret S, Loche M, Mach B, Tartar A, Mouton Y, Capron A. N Engl J Med. 1989;320:251–252. doi: 10.1056/NEJM198901263200415. [DOI] [PubMed] [Google Scholar]
- 17.Reiss P, de Ronde A, Lange J M A, de Wolf F, Dekker J, Debouck C, Goudsmit J. AIDS. 1989;3:227–233. doi: 10.1097/00002030-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Cheingsong-Popov R, Panagiotidi C, Ali M, Bowcock S, Watkins P, Aronstam A, Wassef M, Weber J. AIDS Res Hum Retroviruses. 1990;6:1099–1105. doi: 10.1089/aid.1990.6.1099. [DOI] [PubMed] [Google Scholar]
- 19.Guy B, Rivière Y, Dott K, Regnault A, Kieny M P. Virology. 1990;176:413–425. doi: 10.1016/0042-6822(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 20.Kienzle N, Enders M, Buck M, Siakkou H, Jahn S, Petzold G, Schneweis K E, Bachmann M, Müller W E G, Mueller-Lantzsch N. Arch Virol. 1992;126:293–301. doi: 10.1007/BF01309702. [DOI] [PubMed] [Google Scholar]
- 21.Macreadie I G, Castelli L A, Lucantoni A, Azad A A. Gene. 1995;162:239–243. doi: 10.1016/0378-1119(95)00316-x. [DOI] [PubMed] [Google Scholar]
- 22.Curtain C C, Separovic F, Rivett D, Kirkpatrick A, Waring A J, Gordon L M, Azad A A. AIDS Res Hum Retroviruses. 1994;10:1231–1240. doi: 10.1089/aid.1994.10.1231. [DOI] [PubMed] [Google Scholar]
- 23.Otake K, Fujii Y, Nakaya T, Nishino Y, Zhong Q, Fujinaga K, Kameoka M, Ohki K, Ikuta K. J Immunol. 1994;153:5826–5837. [PubMed] [Google Scholar]
- 24.Fujinaga K, Zhong Q, Nakaya T, Kameoka M, Meguro T, Yamada K, Ikuta K. J Immunol. 1995;155:5289–5298. [PubMed] [Google Scholar]
- 25.Fujii Y, Ito M, Ikuta K. Vaccine. 1993;11:837–847. doi: 10.1016/0264-410x(93)90359-6. [DOI] [PubMed] [Google Scholar]
- 26.Bentin J, Tsoukas C D, McCutchan J A, Spector S A, Richman D D, Vaughan J H. J Clin Immunol. 1989;9:159–168. doi: 10.1007/BF00916944. [DOI] [PubMed] [Google Scholar]
- 27.Gruters R A, Terpstra F G, De Jong R, Van Noesel C J M, Van Lier R A W, Miedema F. Eur J Immunol. 1990;20:1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 28.Clerici M, Shearer G M. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 29.Barcellini W, Rizzardi G P, Borghi M O, Fain C, Lazzarin A, Meroni P L. AIDS. 1994;8:757–762. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Haraguchi S, Good R A, James-Yarish M, Cianciolo G J, Day N K. Proc Natl Acad Sci USA. 1995;92:3611–3615. doi: 10.1073/pnas.92.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collette Y, Dutartre H, Benziane A, Olive D. AIDS. 1996;10:441–442. doi: 10.1097/00002030-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Kaminchik J, Bashan N, Pinchasi D, Amit B, Sarver N, Johnston M I, Fischer M, Yavin Z, Gorecki M, Panet A. J Virol. 1990;64:3447–3454. doi: 10.1128/jvi.64.7.3447-3454.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popovic M, Read-Connole E, Gallo R C. Lancet. 1984;ii:1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- 34.Sundstrom C, Nilsson K. Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 35.Kubin M, Chow J M, Trinchieri G. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- 36.Masood R, Lunardi-Iskandar Y, Moudgil T, Zhang Y, Law R E, Huang C, Puri R K, Levine A M, Gill P S. Biochem Biophys Res Commun. 1994;202:374–383. doi: 10.1006/bbrc.1994.1938. [DOI] [PubMed] [Google Scholar]
- 37.de Waal Malefyt R, Figdor C G, de Vries J E. In: Interleukin-10. de Vries J E, de Waal Malefyt R, editors. Austin, TX: Landes; 1995. pp. 37–52. [Google Scholar]
- 38.Cianciolo G J, Kipnis R J, Snyderman R. Nature (London) 1984;311:515. doi: 10.1038/311515a0. (lett.). [DOI] [PubMed] [Google Scholar]
- 39.Cianciolo G J, Copeland T D, Oroszlan S, Snyderman R. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- 40.Mitani M, Cianciolo G J, Snyderman R, Yasuda M, Good R A, Day N K. Proc Natl Acad Sci USA. 1987;84:237–240. doi: 10.1073/pnas.84.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haraguchi S, Liu W T, Cianciolo G J, Good R A, Day N K. Cell Immunol. 1992;141:388–397. doi: 10.1016/0008-8749(92)90157-k. [DOI] [PubMed] [Google Scholar]
- 42.Haraguchi S, Good R A, Day N K. Immunol Today. 1995;16:595–603. doi: 10.1016/0167-5699(95)80083-2. [DOI] [PubMed] [Google Scholar]
- 43.Chirmule N, Oyaizu N, Saxinger C, Pahwa S. AIDS. 1994;8:733–739. doi: 10.1097/00002030-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Collette Y, Chang H-L, Cerdan C, Chambost H, Algarte M, Mawas C, Imbert J, Burny A, Olive D. J Immunol. 1996;156:360–370. [PubMed] [Google Scholar]
- 45.Werner T, Ferroni S, Saermark T, Brack-Werner R, Banati R B, Mager R, Steinaa L, Kreutzberg G W, Erfle V. AIDS. 1991;5:1301–1308. doi: 10.1097/00002030-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J-S, Kaufman J, Palmer I, Stahl S J, Wingfield P T. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 47.Lee C-H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 48.Nunn M F, Marsh J W. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frankel A D, Pabo C O. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 50.Torres B A, Johnson H M. Biochem Biophys Res Commun. 1994;200:1059–1065. doi: 10.1006/bbrc.1994.1557. [DOI] [PubMed] [Google Scholar]
- 51.Akridge R E, Oyafuso L K M, Reed S G. J Immunol. 1994;153:5782–5789. [PubMed] [Google Scholar]
- 52.Saville M W, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Blood. 1994;83:3591–3599. [PubMed] [Google Scholar]
- 53.Stewart J P, Rooney C M. Virology. 1992;191:773–782. doi: 10.1016/0042-6822(92)90253-l. [DOI] [PubMed] [Google Scholar]
- 54.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. J Immunol. 1991;147:2713–2716. [PubMed] [Google Scholar]
- 55.Clerici M, Wynn T A, Berzofsky J A, Blatt S P, Hendrix C W, Sher A, Coffman R L, Shearer G M. J Clin Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]