Abstract
Membrane composition serves to identify intracellular compartments, signal cell death, as well as to alter a cell’s electrical and physical properties. Here we use amperometry to show that supplementation with the phospholipids phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), and phosphatidylserine (PS) can alter several aspects of exocytosis. Changes in the amperometric peak shape derived from individual exocytosing vesicles reveal that PC slows expulsion of neurotransmitter while PE accelerates expulsion of neurotransmitter. Amperometry data reveal a reduced amount of catecholamine released per event from PC-treated cells while electron micrographs indicate the vesicles in these cells are 50% larger than controls, thus providing evidence of pharmacological changes in vesicle concentration. Addition of SM appears to affect the rate of fusion pore expansion, indicated by slower peak rise times, but does not affect decay times or quantal size. Addition of PS results in a 1.7-fold increase in the number of events elicited by high-K+ depolarization. Electron micrographs of PS-treated cells suggest that increased vesicle recruitment underlies enhanced secretion. We did not observe any effect of phosphatidylinositol (PI) treatment. Together these data suggest that differences in membrane composition affect exocytosis and might be involved in mechanisms of cell function controlling the dynamics of communication via exocytosis.
Keywords: Dopamine, Membrane phospholipid, Exocytosis, Plasticity, PC12 cell
1. Introduction
Exocytosis is a fundamental cellular mechanism for the expulsion of signal molecules from vesicles, such as catecholamines and peptides. Minor changes in the kinetics of exocytosis have a dramatic impact on the function of synapses (Choi et al., 2000). Such changes may underlie the altered neuronal activity associated with phenomena like learning and memory, or could reflect deficits associated with neurodegenerative diseases, such as Parkinson’s disease (Pothos, 2002).
While many of the proteins involved in exocytosis have been characterized and the role each plays in regulating exocytosis is becoming more clear, it is possible that membrane phospholipids also play a role in changing the exocytosis process. Exo/endocytosis proteins have been shown to interact with phospholipids, and the strength of the interaction is headgroup specific (Kohler et al., 1997; Popoli et al., 1997; Quetglas et al., 2002; Wenk and De Camilli, 2004; Hui et al., 2005). Furthermore, the activity of many important exo/endocytosis proteins is impaired in vitro when specific lipid species are absent from the preparation.
Membrane composition depends on cell type and organelle (Devaux, 1991) and membrane lipid composition is actively regulated. Membrane compartments have different amounts of phospholipid species and exhibit an asymmetrical distribution between the inner and outer leaflets. In general, the majority of the aminophospholipids, phosphatidylethanolamine and phosphatidylserine, preferentially segregate to the inner leaflet while the cholinephospholipids, phosphatidylcholine and sphingomyelin, segregate to the outer leaflet (Devaux, 1991; Devaux and Morris, 2004). Exocytosis quickly redistributes phospholipids between the inner and outer leaflets (Demo et al., 1999; Lee et al., 2000) and the amount of secretion is related to the amount of membrane lipid redistribution (Demo et al., 1999). The asymmetric distribution of lipids with respect to the inner and outer leaflets is essential for exocytosis, as this process is inhibited in homogeneous membranes (Kato et al., 2002). Therefore, it would seem that membrane composition is an important point of regulation for exocytosis and such observations lend credence to the view that lipids, the major component of membranes, may play some kind of regulatory role in exocytosis.
One hypothesis of how phospholipid species influence exocytosis is that the concentration of cone-shaped-lipids (as determined by the size of the headgroup relative to the tails) facilitates a transition of the membrane from a planar orientation to the highly curved one necessary to initiate fusion of the plasma membrane with the vesicle membrane. In support of this, the cone-shaped lipid 2-AEP appears to preferentially segregate to the conjugation fusion zone of mating Tetrahymena (Ostrowski et al., 2004). This conjugation zone is comprised of many dense lipidic fusion pores, microstructures whose formation would be facilitated by lipids that promote spontaneous curvature, like 2-AEP. Therefore, we sought to test whether variously shaped species could alter the kinetics of vesicle fusion during stimulated exocytosis in a manner predicted by lipid structures.
We have employed amperometry to monitor exocytosis in the neuron-like immortalized PC12 cell line (Greene and Tischler, 1976) supplemented with various phospholipid species. Stimulated PC12 cells release the easily oxidized neurotransmitter dopamine, which is detected as current peaks by a carbon fiber electrode placed on the cell surface (Chen et al., 1994). Each peak corresponds to the amount of dopamine from a single fusing vesicle and the amount can be quantified by Faraday’s Law (Wightman et al., 1991). The shape of the current peak provides information about the rate of fusion pore expansion (Schroeder et al., 1996). With cells supplemented with different phospholipid species, we find that PC, PE and SM affect the kinetics of exocytosis, PS increases the frequency of exocytosis, and PC reduces quantal size but increases vesicle volume. From this we assert that PC, PE, and SM might play a role in changing the rates of formation and expansion of the fusion pore. It appears that PS participates in recruitment of vesicles to the membrane surface and may interact with proteins that govern the probability of a successful release event. The effects of PC might be considered useful in pharmacological treatment of the efficacy of dopamine release.
2. Results
2.1. PS increases the frequency of vesicle fusion events
We observe a 70% increase in the number of K+-stimulated amperometric peaks following 3-day incubation with PS (Figs. 1a-c). Incubation with any other phospholipid did not alter the frequency of events compared to untreated cells tested the same day (data not shown). We used two different procedures incubating cells for 4 days in 10 μM PS compared to 3 days in 100 μM PS. The increase in number of events to 170% of control after incubation with 100 μM PS was not significantly different than that observed after incubation with 10 μM PS(Fig. 1c) where the number of events is 150% of control. The increase in frequency of peaks was observed with each of three successive stimulations 30 s apart.
Fig. 1.

Representative amperometric traces from (a) untreated control and (b) PS-treated cells show that PS treatment results in greater spike frequency following stimulation with elevated potassium (arrows). Inset: representative peak shape of individual spikes. (c) This difference is observed following incubation with 10 uM or 100 uM PS. Gray bars: untreated; Black Bars: PS-treated cells. Data were collected for a period of 30 s following stimulation. Baseline noise was evaluated for 5 s prior to stimulation. For 10 uM PS n = 30 cells (control was n = 28 cells), for 100 uM PS n = 41 cells (control was n = 38 cells).
We then employed electron microscopy to ascertain whether the increased frequency of vesicle fusion events could be explained by changes in the number or localization of vesicles within PS-treated cells. Although the number of vesicles per cell was not different from control, we did observe changes related to where the vesicles were found within the cell (Fig. 2). We counted “morphologically docked” vesicles that appeared adjacent to the membrane in PS-treated cells and concluded that the fraction of the total vesicles that were morphologically docked was significantly lower than that observed in control cells. However, in cells that were examined after being treated with PS, stimulated with potassium ion, and then fixed, we found that the number of vesicles residing adjacent to the membrane was dramatically increased. The initial decrease in docked, unstimulated vesicles in the PS group closely matches the fractions observed with unstimulated PC (Fig. 2) and l-DOPA treated vesicles (unpublished observations), and thus may be a result of enlarged vesicle size (Fig. 5c). In contrast, when cells were fixed during potassium stimulation, the fraction of docked vesicles in the PS-treated group nearly doubles (from 0.17 to 0.32), whereas no significant increase was observed with untreated or PC-treated cells. This suggests that PS may play a role in translocating or retaining cytosolic vesicles to or near the membrane during stimulation.
Fig. 2.
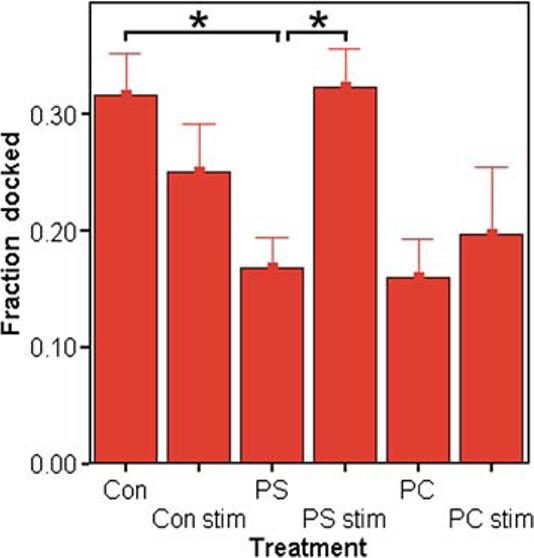
The fraction of morphologically docked vesicles (observed to be adjacent to the membrane) increases after depolarization in PS-treated (100 μM, 3 days) cells (PS-stim), but not in unstimulated (PS) cells (n = 12 to 23 cells per condition; * denotes p ≤ 0.05). Unstimulated PC-treated cells are not significantly different from control (Con; p ≤ 0.07) and do not appear to increase upon stimulation (PC stim). The criteria for counting vesicles was that the distance between the vesicle and the membrane was <50 nm or the vesicle was within a quarter of the vesicle diameter from touching the membrane.
Fig. 5.
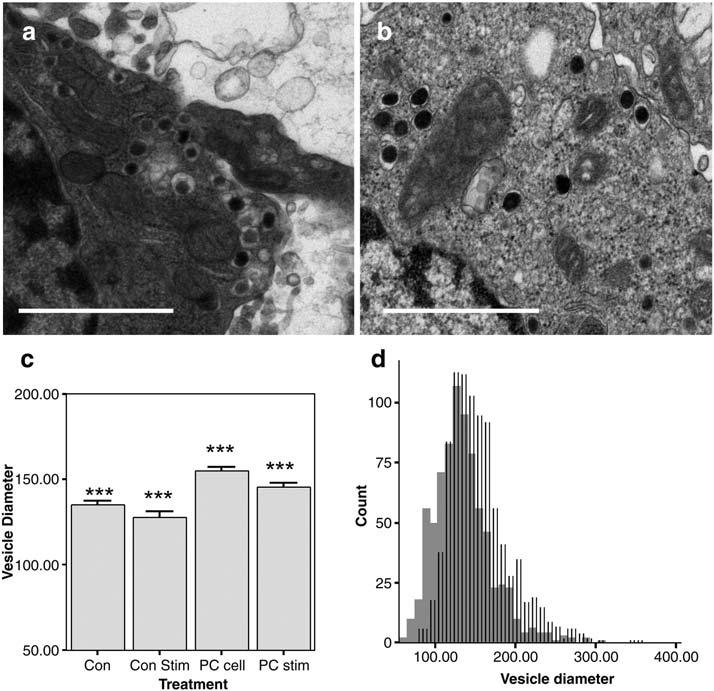
Electron micrographs of vesicles in (a) control and (b) PC enriched cells (100 μM, 3 days); magnification (scale bar = 1 μM). (c) Mean vesicle sizes for control (Con) and PC enriched cells (PC cell). “Con Stim” and “PC Stim” cells were treated with a 5-s stimulation of 100 mM K+ prior to fixation. Following stimulation, vesicles in control and PC cells decrease slightly; n = 780, 580, 911, and 609 vesicles from 14-23 cells per condition were examined; *** indicates that all groups are different at p < 0.001 by ANOVA, Tukey-HSD test. Error bars are S.E.M. (d) Histogram of vesicle diameters illustrates a uniform shift to larger diameters with PC treatment (lines) vs. control (solid).
2.2. PE, SM, and PC affect the kinetics of exocytosis
We measured exocytosis in cells incubated for 3 days with PE, SM, PC, and PI. Unlike PS, no significant changes in the frequency of exocytosis were observed. However, we did observe changes in peak shape following PE, SM, and PC treatment, indicative of altered vesicle fusion kinetics. Fig. 3 displays typical peaks for each treatment and peaks from control cells that for each case were tested on the same day. Of course, there is a great deal of variability from day to day. Thus, importantly, Fig. 4 presents amplitude-normalized traces averaged from all cells per treatment. While PS and PI did not affect vesicle fusion kinetics, treatment with PE decreased the half-width and decay times while increasing the amplitude of release. Treatment with SM appeared to affect the initial stages of vesicle fusion, evident by longer rise times and shallower rise slopes of the peak, but half-width also increased slightly. The effect of treatment with PC contrasted that of PE, decreasing the amplitude and rise time of release, while lengthening decay times and half-widths. Fig. 4 provides a comparative summary of changes in peak parameters for all groups.
Fig. 3.

Representative amperometric current transients from control (gray traces) and phospholipid treated (black traces) cells following treatment (100 μM, 3 days) with five different phospholipids. These were chosen to represent the “typical” response between treated cells. Average data are shown in Fig. 4.
Fig. 4.

Incubation with PE, SM, or PC (100 μM, 3 days) changes the kinetics of individual exocytosis events. (a) Amplitude-normalized mean traces from PE, SM, and PC events demonstrate respective changes in rising and falling portion of peaks. Dotted traces (blue) are from phospholipid-treated cells; solid lines (green) are from control cells. Peaks from each cell were averaged, and the means of these averages were temporally aligned to the point of maximum amplitude to generate the normalized mean trace. (b) Summary plots of changes in half-width (t1/2), 10-90% rise time, 10-90% slope, and decay time (ms) for all treatment groups and their same-day respective controls (n =25-40 cells per group; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 vs. same-day-tested untreated control group by t-test).
2.3. PC reduces quantal size and slows vesicle fusion
The wider, flatter peaks resulting from PC supplementation can be interpreted as the result of a slower rate of vesicle fusion during exocytosis. In addition, we observed a 26% decrease in mean area of amperometric peaks, suggesting that fewer molecules of neurotransmitter were released with each vesicle fusion event. Treatment with the other phospholipids discussed here did not have a statistically significant effect on quantal size. The smaller quantal size (amount of neurotransmitter release detected per exocytosis) after PC treatment could result from a decreased concentration of dopamine in the vesicles, an incomplete “kiss and run” type flux, or from smaller vesicles. Therefore, we analyzed PC-treated vesicles with electron microscopy (Fig. 5). Unexpectedly, we observed a small but statistically significant 15% increase in vesicle diameter (50% increase in volume) in PC-treated cells vs. control (Fig. 5c). In an effort to predict whether only recycling vesicles were able to incorporate excess lipid we then asked whether a subset of vesicles had increased, or whether all vesicles appeared to increase. A histogram of vesicle sizes (Fig. 5d) suggests that the change in vesicle size is uniform, as no subset of enlarged vesicles among the population is observed.
For comparison, we also examined PS-treated cells with TEM. We found a similar, although not statistically significant, 8% increase in vesicle diameter (27% increase in volume) for PS-treated cells (data not shown). It is possible that phospholipid treatment might enlarge vesicles by general incorporation of excess lipid. Because we did not observe a change in quantal size from PS-treated cells despite larger vesicles, these observations suggest that the concentration within vesicles may decrease to compensate for increases in volume.
Because dramatic changes in the fraction of docked vesicles were observed for PS-treated cells following stimulation (Fig. 2), we asked whether vesicle sizes change upon stimulation. For all groups examined (control-stim, PS-stim, and PC-stim), we observed slight but statistically significant decreases in vesicle diameter following K+ stimulation (Fig. 5c). Again, these decreases appear to affect the distribution of vesicle sizes as a whole as no bimodal distributions were observed in histograms of vesicle size.
3. Discussion
A summary of the effects on exocytosis reported here is presented in Table 1. Although we assume that incubation in 100 μM solutions of each lipid increases the lipid content of the cell membranes, we do not know the quantitative change in various membranes. Given that caveat, the most dramatic effects of phospholipid treatment are (a) the PS-induced increase in exocytotic frequency, (b) the PC-induced decrease in quantal size and increase in mean vesicular volume and concomitant increase in time needed for vesicle fusion, (c) the PE-induced increase in the kinetics of vesicle fusion, which can be explained by the hypothesis that cone-shaped lipids facilitate the fusion process, and (d) the slowed vesicle fusion by SM. Possible explanations and implications of each will be examined in turn.
Table 1.
Summary of lipid-induced effects on exocytosis, manifested as changes in amperometric peak characteristics
| PS | PI | PE | SM | PC | |
|---|---|---|---|---|---|
| No. of events | ●+ | ||||
| Total secretion | ⊚+ | ||||
| Quantal size | ⊚- | ||||
| Amplitude | ○+ | ○- | ●- | ||
| Half width | ⊚- | ⊚+ | ●+ | ||
| Rise time | ○+ | ||||
| 10-90 slope | ●- | ●- | |||
| Decay time | ●- | ●+ |
p≤0.05
p≤0.01
p≤0.001 (ANOVA, Tukey-HSD test, α≤0.05).
3.1. Added PS might increase exocytosis frequency by protein-lipid interactions
The observed increase in the number of depolarization-induced exocytosis events following PS treatment indicates that PS may be a limiting factor in vesicle recruitment or fusion. PS binds several calcium-sensitive proteins implicated in exocytosis, including synaptotagmin (SYT) (Popoli et al., 1997), VAMP (Quetglas et al., 2002; Duman et al., 2004), t-SNAREs [syntaxin1A and SNAP-25] (Wagner and Tamm, 2001), annexin (Kohler et al., 1997), the dynamins (Burger et al., 2000; Accola et al., 2002) and amphiphysin (Wenk and De Camilli, 2004). The activity of any one of these proteins may be enhanced in the presence of excess PS, increasing the probability of fusion. Enhanced recruitment of vesicles from the non-releasable reserve pool to the readily releasable pool following stimulation could also partly explain the observed increase in stimulated events, as electron micrographs reveal a stimulus-dependent increase in the fraction of “morpholo-gically docked” vesicles (Fig. 2). This stimulus-dependent increase in fraction of docked vesicles was not observed in control or PC-treated cells, further suggesting that PS may act in part by facilitating vesicle recruitment via a specific protein-headgroup interaction. However, recruitment alone cannot explain the increase. The increased frequency of events is observed during the first and during each subsequent stimulation, indicating that PS treatment increases the probability of exocytosis in a stimulus-independent manner (Fig. 1b). This further implicates some form of underlying interaction between PS and a protein that plays a critical, rate-determining role in initiating exocytosis.
3.2. Added PC decreases quantal size while increasing vesicular volume and slowing the dynamics of exocytosis
Table 2 provides more detail on the physical differences between the phospholipids studied. The acyl chain composition of our PC was identical to that of PI, which had no effect on exocytosis. Therefore, the properties of the headgroup appear to govern the effects observed. The PC headgroup is very strongly hydrated compared to the other headgroups, which we speculate could explain why the kinetics of fusion are slower in PC-treated cells. Protein-PC interactions could also play a role, although few proteins involved in exocytosis show an affinity for PC over PS in vitro, as PC is found predominantly on the exoplasmic membrane.
Table 2.
Summary of property in each phospholipid
| PS | PI | PE | SM | PC | |
|---|---|---|---|---|---|
| Charge | Negative | Negative | Neutral | Neutral | Neutral |
| Ratio of saturated/ unsaturated | 0.95 | 0.68 | 0.82 | 1 | 0.69 |
| Membrane localization | Inner | Depends on cell organelle | Inner | Outer | Outer |
| Shape | Cylinder | Cylinder | Cone | Cylinder | Cylinder |
While we cannot yet attribute the effects observed following PC treatment to a definitive mechanism, these effects may be important to the pathology of one or more neurodegenerative diseases. Lee and coworkers report that MPP+ and S-adenosyl-l-methionine facilitate the conversion of PE to PC and lysopho-sphatidylcholine (lysoPC) in rodent models of Parkinson’s disease (Lee and Charlton, 2001; Lee et al., 2005a,b). Nitsch and coworkers report a significant decrease in the amount of PC and PE in post-mortem brain extracts from Alzheimer disease patients (Nitsch et al., 1992). Here we report that addition of PC decreases quantal size and increases vesicle volume while significantly reducing the rate of neurotransmitter extrusion from vesicles. It is interesting to speculate that these effects of PC on vesicle and quantal size might have specific impact on development of treatments and prevention of Parkinson’s Disease. Oxidative stress has been implicated in the events leading to cell death in this disease (Jenner, 2003). l-DOPA treatment increases dopaminergic activity by increasing quantal size (Kozminski et al., 1998). Increased DA is thought to lead to oxidative stress and, in the presence of fewer cells to actively remove DA, this might increase the progression of the disease. Reduction of quantal size in synaptic terminals following PC should limit oxidative stress. A simultaneous increase in vesicle volume following PC treatment should lead to a larger release volume of transmitter during each quantal event. A larger volume released into the synapse should act to increase DA transmission by impacting a larger number of active zones. Thus, increasing vesicle size without increasing DA levels might serve as an effective treatment to increase dopaminergic activity and alleviate the disease symptoms while not increasing the oxidative stress on the surrounding cells. This could be especially important in prevention of Parkinson’s disease where limiting dopamine quantal size with PC should reduce cell loss owing to normal dopamine-related oxidative stress. It is possible that the phenomena reported here are related to a recent report that a phospholipid-based drug formulation can reduce neurodegeneration and abrogate oxidative stress in the 6-OHDA rat model of Parkinson’s disease leading to functional recovery in vivo (Fitzgerald et al., 2005).
3.3. Adding PE leads to faster individual release events
The structure of PE consists of two fatty acids attached to the relatively small head group ethanolamine. As the tails occupy more space than the head group, PE has a conical shape with the head group at the vertex. Cone-shaped lipids induce inward curvature of membranes when concentrated in the inner surface of lipid bilayers, where they are predominantly found in cells (Devaux and Morris, 2004). Inward curvature reduces the energy required for membrane-to-vesicle fusion, and therefore would likely increase the rate of fusion pore formation, expansion, and neurotransmitter extrusion. Indeed, this model explains our observations quite well, as incubation with the one cone-shaped lipid increased the kinetics of release while this effect was not observed for the four cylindrical lipids tested.
It seems possible that previously observed changes in enzymatic pathways can be attributed to PE effects in the membrane. The observed abbreviated fusion events following PE treatment closely resemble those induced by treatment with PMA, a PKC activator (Burgoyne et al., 2001; Graham et al., 2002). Recently, Deli and Kiss reported that PKC-α directly regulates PE hydrolyzation via a novel PE-specific phospholipase-D (Deli and Kiss, 2000). Lipids commonly serve as the substrate for regulatory enzymes to produce signaling molecules. It is possible that exocytosis could be biophysically modulated during the process of rapid lipid-to-signal molecule interconversion by bulk removal or addition of PE.
3.4. Added SM appears to slow vesicle opening
We observe that SM specifically affects the rising portion of peaks, indicative of slower fusion pore opening and expansion (Graham and Burgoyne, 2000). SM and cholesterol apparently form regions of reduced fluidity within membranes (lipid rafts), but the function of these microdomains remains unclear (Salaun et al., 2004). Recently Salaun and others have shown that SNARE complexes associate with lipid rafts in membranes to reduce exocytosis (Salaun et al., 2005a,b). Although incubation with SM does not alter the number of stimulated events, changes in the kinetics of exocytosis have been observed that could be explained by interference from lipid rafts.
3.5. Implications
An important implication of the results presented here is that changes in membrane PC and PS content might contribute to presynaptic plasticity, possibly through lipid-protein interactions. Two other lipids tested, PE and SM, appear to influence the kinetics of neurotransmitter extrusion from vesicles, by currently unknown mechanisms. PI did not appear to affect exocytosis. These preliminary observations provide new evidence for a critical role of membrane mechanics based on phospholipid diversity in modulating the dynamics of release via exocytosis. It is possible that changes in phospholipid composition in active zones could form a mechanism for one form of changing neuronal communication by altering the rate of individual exocytosis events.
4. Experimental procedures
4.1. Cell culture
PC12 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained as described previously (Kozminski et al., 1998). PC12 cells were grown on mouse collagen-IV-coated dishes (Becton Dickinson, Bedford, MA) in RPMI-1640 medium supplemented with 10% equine and 5% fetal bovine serum in a 7% CO2 atmosphere, 37 °C. Cells were subcultured approximately every 7-9 days or when confluency was reached. Except where noted (Fig. 1c), we chose to incubate cells with 100 μM of each phospholipid ((Avanti Polar Lipids, Alabaster, AL) PS #830032, brain; PI #840044, soybean; PE #831118, egg; SM 860061, egg; PC #840054, soybean) in 0.1% DMSO for 3 days before amperometry measurements, starting at 3 days after subculturing. Control sister cultures were plated and maintained separately under identical conditions for the same duration with media supplemented with vehicle (0.1% DMSO (Sigma, St. Louis, MO) starting 3 days after subculturing.
4.2. Amperometry experiments
4.2.1. Electrodes
Carbon fiber microelectrodes (5 μm diameter) were constructed as described previously (Pothos et al., 1998) and back-filled with 3 M KCl. Electrode tips were polished at a 45° angle on a diamond dust-embedded micropipette beveling wheel (Model BV-10; Sutter Instrument Co., Novato, CA). Cyclic voltammograms were generated for each electrode in a nitrogen-saturated 0.1 mM dopamine solution (in 0.1 M Na2HPO4, pH 7.4), and only electrodes with stable I-E curves were used.
4.2.2. Solutions
Cells were bathed in saline (150 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, 5 mM glucose, 10 mM HEPES) during amperometry recordings. The elevated 100 mM K+ saline used to depolarize cells was osmotically balanced by reducing the NaCl concentration to 55 mM.
4.2.3. Experiment setup
Electrodes were held at +700 mV vs. a locally constructed sodium-saturated calomel reference electrode using a commercially available patch-clamp instrument (Axopatch 200B; Axon Instruments, Foster City, CA) configured as described previously (Borges et al., 1997). Cells were prepared for experiments as described previously (Colliver et al., 2000a). Measurements from single, isolated cells were collected on day 6 after subculturing. Control and phospholipid-treated cells were assayed alternately on the same day of experimentation. Typically 2-4 cells per culture dish were used until measurements from at least 25 cells were collected for each condition. When possible, the same electrode was used to measure exocytosis from alternating control and phospholipid treated PC12 cells. This approach minimizes variability between measurements. Each cell was stimulated three times (5 s, 20 psi pulses (Picospritzer II; General Valve Instruments, Fairfield, NJ)) with 100 mM K+ at 30 s intervals on an inverted microscope stage heated to 37 ± 1 °C (Bionomic System, 20/20 Technology, Inc., Wilmington, NC). Cells responding with fewer than 6 events per stimulation were excluded.
4.2.4. Data acquisition and analysis
The output was digitized at 5 kHz and filtered at 2 kHz using an internal four-pole low-pass Bessel filter. Data were displayed in real time (Axoscope 8.1.0.07; Axon Instruments, Foster City, CA) and stored to the computer with no subsequent filtering. Amperometric peaks were measured using Mini Analysis software (Synaptosoft, Decatur, GA) and the peak characteristics of area (Q, pC), half-width (t1/2, ms), 10-90% peak rise time (rise, ms), and decay time (from Imax to 0.33% of max, ms) were determined. Signals were designated as spikes if their Imax values exceeded five times the RMS noise of a 1 s portion of stable baseline recorded prior to the beginning of each experiment. All peaks identified by the program were inspected visually, and poorly fit peaks were either manually recalculated or excluded from data sets due to the presence of complex traits (exhibiting multiple peak maxima) or noise interference.
4.3. Transmission electron microscopy
PC12 cells were rinsed with PBS, pH 7.4, and treated with Ca2+- and Mg2+-free 0.05% trypsin-EDTA (Invitrogen, Gaithersburg, MD) for 30 s. The trypsin-EDTA solution was removed and the cells were dispersed into solution by flushing them off the culturing substrate with PBS. Single-cell suspensions were transferred to microfuge tubes and pelleted at 100×g for 10 min. Some cell pellets were exposed to 100 mM K+ (identical to that used for amperometry experiments) for 5 s immediately prior to the addition of Karnovsky’s fixative, overnight at 4°C (Karnovsky, 1968). Finally, the pellets were prepared for transmission electron microscopy (TEM) as described previously (Colliver et al., 2000a) and viewed on a transmission electron microscope (1200EXII; JEOL, Peabody, MA) at 80 kV.
Vesicle diameters in TEM images were performed using ImagePro Plus (ver 4.5.1.28, Media Cybernetics, Inc. Silver Spring, MD). Mean vesicle diameters were determined for 6 groups: control, PS, and PC with and without brief K+-stimulation immediately prior to fixing. Only vesicles in which a dense core could be clearly identified were measured, and images were not corrected for effect of plane of section on vesicle sizes (Coupland, 1968). Therefore, measured changes in diameter reflect relative changes in vesicle morphology.
4.4. Statistical analysis
To ensure that cells with a large number of events would not be overrepresented within a treatment group, we compared treatment group means derived from mean parameters of each cell (e.g. each cell contributes one average data point rather than n individual data points) (Colliver et al., 2000b). All data were tested for significant differences using the t test and a one-way ANOVA with Tukey-HSD post hoc analysis. The Tukey analysis compares all groups to each other with alpha = 0.05 strictly controlled (SPSS, Ver 10.1, SPSS, Inc., Chicago, IL). All values are reported as the mean± S.E.M.
Acknowledgments
This work was supported, in part, by a grant from the National Institutes of Health.
Abbreviations
- DA
dopamine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- PC
phosphatidylcholine
- SM
sphingomyelin
- 2-AEP
2-aminoethylphosphonolipid
- PC12
pheochromoytoma
- PI
phosphatidylinositol
REFERENCES
- Accola MA, Huang B, Al Masri A, McNiven MA. The antiviral dynamin family member, MxA, tubulates lipids and localizes to the smooth endoplasmic reticulum. J. Biol. Chem. 2002;277:21829–21835. doi: 10.1074/jbc.M201641200. [DOI] [PubMed] [Google Scholar]
- Borges R, Travis ER, Hochstetler SE, Wightman RM. Effects of external osmotic pressure on vesicular secretion from bovine adrenal medullary cells. J. Biol. Chem. 1997;272:8325–8331. doi: 10.1074/jbc.272.13.8325. [DOI] [PubMed] [Google Scholar]
- Burger KN, Demel RA, Schmid SL, de Kruijff B. Dynamin is membrane-active: lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry. 2000;39:12485–12493. doi: 10.1021/bi000971r. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Fisher RJ, Graham ME, Haynes LP, Morgan A. Control of membrane fusion dynamics during regulated exocytosis. Biochem. Soc. Trans. 2001;29:467–472. doi: 10.1042/bst0290467. [DOI] [PubMed] [Google Scholar]
- Chen TK, Luo G, Ewing AG. Amperometric monitoring of stimulated catecholamine release from rat pheochromocytoma (PC12) cells at the zeptomole level. Anal. Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at ‘silent synapses’. Nat. Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-Mediated changes in quantal size and vesicular volume. J. Neurosci. 2000a;20:5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliver TL, Hess EJ, Pothos EN, Sulzer D, Ewing AG. Quantitative and statistical analysis of the shape of amperometric spikes recorded from two populations of cells. J. Neurochem. 2000b;74:1086–1097. doi: 10.1046/j.1471-4159.2000.741086.x. [DOI] [PubMed] [Google Scholar]
- Coupland RE. Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature. 1968;217:384–388. doi: 10.1038/217384a0. [DOI] [PubMed] [Google Scholar]
- Deli E, Kiss Z. Protein kinase C-stimulated formation of ethanolamine from phosphatidylethanolamine involves a protein phosphorylation mechanism: negative regulation by p21 Ras protein. Arch. Biochem. Biophys. 2000;377:171–177. doi: 10.1006/abbi.2000.1768. [DOI] [PubMed] [Google Scholar]
- Demo SD, Masuda E, Rossi AB, Throndset BT, Gerard AL, Chan EH, Armstrong RJ, Fox BP, Lorens JB, Payan DG, Scheller RH, Fisher JM. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry. 1999;36:340–348. doi: 10.1002/(sici)1097-0320(19990801)36:4<340::aid-cyto9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Devaux PF, Morris R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 2004;5:241–246. doi: 10.1111/j.1600-0854.2004.0170.x. [DOI] [PubMed] [Google Scholar]
- Duman JG, Lee E, Lee GY, Singh G, Forte JG. Membrane fusion correlates with surface charge in exocytic vesicles. Biochemistry. 2004;43:7924–7939. doi: 10.1021/bi036304q. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P, Harris D, Crotty S, Harkin A, Mandel A, Bolton AE, Sullivan AM, Nolan Y. Treatment with a novel phospholipid-based drug formulation inhibits 6-hydroxydopamine-induced p38 activation in rat substantia nigra; Society for Neuroscience Annual Meeting; Washington, D.C.. 2005. [Google Scholar]
- Graham ME, Burgoyne RD. Comparison of cysteine string protein (Csp) and mutant alpha-SNAP overexpression reveals a role for csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J. Neurosci. 2000;20:1281–1289. doi: 10.1523/JNEUROSCI.20-04-01281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, O’Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U. S. A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5210–5214. doi: 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P.Oxidative stress in Parkinson’s disease Ann. Neurol 200353Suppl 3S26–S36.(discussion S36-28) [DOI] [PubMed] [Google Scholar]
- Karnovsky M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1968;27:137A–138A. [Google Scholar]
- Kato N, Nakanishi M, Hirashima N. Transbilayer asymmetry of phospholipids in the plasma membrane regulates exocytotic release in mast cells. Biochemistry. 2002;41:8068–8074. doi: 10.1021/bi016022v. [DOI] [PubMed] [Google Scholar]
- Kohler G, Hering U, Zschornig O, Arnold K. Annexin V interaction with phosphatidylserine-containing vesicles at low and neutral pH. Biochemistry. 1997;36:8189–8194. doi: 10.1021/bi9703960. [DOI] [PubMed] [Google Scholar]
- Kozminski KD, Gutman DA, Davila V, Sulzer D, Ewing AG. Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat pheochromocytoma (PC12) cells. Anal. Chem. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- Lee ES, Charlton CG. 1-Methyl-4-phenyl-pyridinium increases S-adenosyl-l-methionine dependent phospholipid methylation. Pharmacol. Biochem. Behav. 2001;70:105–114. doi: 10.1016/s0091-3057(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Lee D, Hirashima N, Kirino Y. Rapid transbilayer phospholipid redistribution associated with exocytotic release of neurotransmitters from cholinergic nerve terminals isolated from electric ray Narke japonica. Neurosci. Lett. 2000;291:21–24. doi: 10.1016/s0304-3940(00)01365-3. [DOI] [PubMed] [Google Scholar]
- Lee ES, Soliman KF, Charlton CG. Lysophosphatidylcholine decreases locomotor activities and dopamine turnover rate in rats. Neurotoxicology. 2005a;26:27–38. doi: 10.1016/j.neuro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Lee ES, Chen H, Charlton CG, Soliman KF. The role of phospholipid methylation in 1-methyl-4-phenyl-pyridinium ion (MPP(+))-induced neurotoxicity in PC12 cells. Neurotoxicology. 2005b doi: 10.1016/j.neuro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski SG, Van Bell CT, Winograd N, Ewing AG. Mass spectrometric imaging of highly curved membranes during Tetrahymena mating. Science. 2004;305:71–73. doi: 10.1126/science.1099791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Venegoni A, Buffa L, Racagni G. Ca2+/ phospholipid-binding and syntaxin-binding of native synaptotagmin I. Life Sci. 1997;61:711–721. doi: 10.1016/s0024-3205(97)00536-5. [DOI] [PubMed] [Google Scholar]
- Pothos EN. Regulation of dopamine quantal size in midbrain and hippocampal neurons. Behav. Brain. Res. 2002;130:203–207. doi: 10.1016/s0166-4328(01)00419-3. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Przedborski S, Davila V, Schmitz Y, Sulzer D. D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. J. Neurosci. 1998;18:5575–5585. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetglas S, Iborra C, Sasakawa N, De Haro L, Kumakura K, Sato K, Leveque C, Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, Gould GW, Chamberlain LH. Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J. Biol. Chem. 2005a;280:19449–19453. doi: 10.1074/jbc.M501923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, Gould GW, Chamberlain LH. The SNARE proteins SNAP-25 and SNAP-23 display different affinities for lipid rafts in PC12 cells. Regulation by distinct cysteine-rich domains. J. Biol. Chem. 2005b;280:1236–1240. doi: 10.1074/jbc.M410674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TJ, Borges R, Finnegan JM, Pihel K, Amatore C, Wightman RM. Temporally resolved, independent stages of individual exocytotic secretion events. Biophys. J. 1996;70:1061–1068. doi: 10.1016/S0006-3495(96)79652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner ML, Tamm LK. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr., Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]


