Abstract
OBJECTIVE
To investigate whether variable coding sequence protein A1 (Vcsa1) is down-regulated in rat models of diabetes and ageing, and to investigate the role of Vcsa1 in erectile function, as Vcsa1 is the most down-regulated gene in the corpora of a rat model of neurogenic erectile dysfunction (ED).
MATERIALS AND METHODS
Quantitative reverse-transcriptase polymerase-chain reaction was used to determine Vcsa1 expression in the corpora of rats in three models of ED, i.e. streptozotocin-induced diabetes, retired breeder (old), and neurogenic (bilaterally ligated cavernosal nerves), and in control rats. To confirm a physiological role of Vcsa1 in erectile function, we carried out gene transfer studies using a plasmid in which Vcsa1 was expressed from a cytomegalovirus promoter (pVAX-Vcsa1). This plasmid was injected intracorporally into old rats, and the effect on physiology of corporal tissue was analysed by intracorporal/blood pressure (ICP/BP) measurement and histological analysis, and compared with the effects of a positive control plasmid (pVAX-hSlo, which we previously reported to restore erectile function in diabetic and ageing rats) and a negative control plasmid (pVAX).
RESULTS
In each rat model of ED there was a significant down-regulation of the Vcsa1 transcript of at least 10-fold in corporal tissue. Remarkably, intracorporal injection with 80 μg pVAX-Vcsa1 caused priapism, as indicated by visible prolonged erection, histological appearance, and elevated resting ICP/BP. Lower doses of pVAX-Vcsa1 (5 and 25 μg) increased ICP/BP over that in untreated controls.
CONCLUSION
These results show that Vcsa1 has a role in erectile function and might be a molecular marker for organic ED. The role of Vcsa1 in erectile function suggests that it could represent a novel therapeutic target for treating ED.
Keywords: Vcsa1, SMR-1, diabetes, ageing, erectile dysfunction, gene transfer, priapism
INTRODUCTION
Recently, variable coding sequence protein A1 (Vcsa1, also known as submandibular rat 1 protein, SMR-1) was shown to be one of the most down-regulated genes in the corpora of rats with a neurogenic model of erectile dysfunction (ED; bilaterally ligated cavernosal nerve, CN) [1]. Vcsa1 encodes a precursor protein that gives rise to three peptide hormones [2]. Several roles for Vcsa1 have been proposed. One of its peptide products, sialorphin, was shown to modulate male sexual behaviour in the rat [3]. Other studies showed that sialorphin is an inhibitor of rat membrane-bound neutral endopeptidase [4] with analgesic activity, and binding studies suggested a link between the circulating peptides and mineral transport [5]. There is considerable sexual dimorphism in the regulation of Vcsa1 gene expression; androgens cause a 100- to 500-fold higher expression of mature peptide hormone levels in adult males compared with adult females [6].
A wide range of risk factors contribute to the development of ED that, depending on the cause, can be broadly classified as organic, psychogenic or mixed [7]. Two of the most common risk factors for organic ED are diabetes and ageing [8]. Diabetic men are three times as likely to develop ED as men with no diabetes, and men aged 50–90 years have 10 times the risk of ED than those <50 years of age [9]. It might be expected that different types of ED with overlapping pathophysiological mechanisms have common biochemical pathways contributing to ED. Resolution of these pathways could identify potential therapeutic targets [10]. Microarray analysis is a useful tool for monitoring overall changes in gene expression with the onset of diabetes. However, to date these types of studies have served only to highlight that ED involves changes in a diverse set of molecular pathways. Not unexpectedly, the genes that are most altered in expression in one model of ED (such as diabetes [11]) differ in another (such as after radical prostatectomy [1]).
Our approach has been to try to identify a marker gene that has changed in expression in several models of ED. Our efforts focused on Vcsa1, because this was recently shown to be one of the most down-regulated genes in the corpora of rats with a neurogenic model of ED [1]. Because, in the present study, quantitative real-time PCR showed that Vcsa1 was down-regulated in both ageing and streptozotocin (STZ)-induced diabetic rats with ED, we wanted to show the involvement of this gene in erectile function. Therefore, we performed intracorporal gene transfer experiments with a vector expressing Vcsa1. Previous work showed the validity of this approach for evaluating the role of the Slo gene (which encodes the α-subunit of the Maxi-K ion channel) [12, 13] in erectile function, and has led to the suggestion that gene transfer of Slo could be used for treating ED. The use of gene transfer of Slo presently is undergoing evaluation in human clinical phase I trials [14].
MATERIALS AND METHODS
The animal model for diabetes used in these experiments was STZ-induced diabetes in male rats (essentially as described in [15]). Routinely, diabetes was induced in F-344 rats (Taconic Farms, Germantown, NY, USA; 8–10 weeks old; weight 200–240 g) by an i.p. injection with STZ (35 mg/kg) dissolved in citrate buffer (0.6 M citric acid/0.08 M Na2HPO4; pH 4.6). Control rats received an injection of vehicle only. STZ-diabetic rats had blood glucose levels of ≥250 mg/dL and urine glucose levels of ≥1000 mg/dL. At 1 week or 2 months after the onset of diabetes, the rats were analysed for intracorporal/blood pressure (ICP/BP) response and then killed by putting them in a CO2 chamber; tissues of interest (corpora, bladder, urethra and ureter) were immediately flash-frozen in liquid nitrogen and were stored at −70 °C until RNA preparation. The numbers of rats used are shown in Table 1.
TABLE 1.
Measurement of erectile function (ICP/BP) in control, 1 week STZ-induced diabetic, 2 month STZ-diabetic, young and old rats
| Mean (sd) ICP/BP
|
||||
|---|---|---|---|---|
| Group | N | Basal | CN stimulation 0.75 mA | CN stimulation 4 mA |
| Control | 4 | 0.063 (0.025) | 0.58 (0.12) | 0.62 (0.166) |
| Diabetic | ||||
| 1 week | 4 | 0.11 (0.023) | 0.55 (0.03) | 0.60 (0.15) |
| 2 months | 5 | 0.023 (0.023) | 0.34 (0.20)* | 0.46 (0.12)* |
| Young | 7 | 0.1 (0.057) | 0.60 (0.14) | 0.63 (0.10) |
| Old | 6 | 0.086 (0.057)† | 0.21 (0.18)† | 0.25 (0.16)† |
P < 0.5 vs control rats, one-way anova;
P < 0.5 vs young rats, one-way anova.
For the ageing rat model, experiments were carried out on young male Sprague–Dawley rats (4–5 months old, weighing ≈275 g) and old ‘retired breeder’ male Sprague-Dawley rats (9–10 months, weighing >500 g, essentially as described in [13]). Rats were analysed for ICP/BP response and were then killed, and the relevant tissues removed and stored until RNA preparation as described above. The numbers of rats used are also shown in Table 1.
The neurogenic ED model rats had bilaterally ligated CNs; four 120-day-old rats had open surgical bilateral CN ligation as previously described [1]. CNs were identified bilaterally on the lateral aspect of the prostate. A portion of the nerve on each side then was sharply dissected free of the surrounding tissues. The CN was directly electrostimulated on each side at 6 mA using bipolar hook-electrodes. The quality of the erections was noted visually on a three-point scale after stimulation of the CN on each side of the prostate (NE = no erection, PE = partial erection, FE = full erection). The CN on each side was then ligated at the most proximal point of the isolated portion using a 3/0 silk tie. The operative site was closed and the rats were allowed to recover. After 9 days, the second open surgical procedure was performed. Again, CNs were identified bilaterally and the ligated portion of each CN was dissected free of the surrounding tissues. The dissection was carried ≈1 cm proximal to the point of ligation. Each CN was electrostimulated proximal to the point of ligation using the same stimulation as used during the first operation; there was no erectile response in any of the rats.
All study protocols were approved by the Animal Use Committee at the Albert Einstein College of Medicine.
For cavernosometry, determining the ICP response to stimulation of the CN, the rats were anaesthetized with pentobarbital sodium (35 mg/kg i.p.). An incision was made in the perineum, and a ‘window’ was made in the ischiocavernosus muscle to expose the corpus cavernosum. The CNs were identified adjacent to the prostate gland. The CN was directly electrostimulated with a delicate stainless steel bipolar hook-electrode attached to a multijointed clamp. Each probe was 0.2 mm in diameter and the two poles were separated by 1 mm. Monophasic rectangular pulses were delivered by a signal generator (custom-made and with built-in constant-current amplifier). The stimulation parameters were: frequency, 20 Hz; pulse width, 0.22 ms; duration, 1 min; current, 0.75 and 4 mA. Changes in ICP and systemic BP were recorded at each intensity of stimulation. The mean ICP/BP, sd and anova were calculated for each treatment group.
For the gene transfer experiments, vectors/ plasmids were microinjected into the rat corporal tissue, essentially as previously described [12, 13, 15]. Briefly, the rats were anaesthetized with pentobarbital sodium (35 mg/kg i.p). An incision was made through the perineum, the corpus spongiosum was identified, and a ‘window’ was made in the corpus spongiosum to identify the corpus cavernosum. All microinjections, using an insulin syringe, consisted of a bolus injection of naked plasmid DNA into the corporal tissue. The final volume of all microinjections was 150 μL. The plasmid pVAX is commercially available (Invitrogen, Carlsbad, CA, USA). Construction of the plasmid pVAX-hSlo was described previously [13]. pVAX-Vcsa1 was generated by PCR amplification of total cDNA from rat corpora using the forward primer 5′-CAAGGGGCTACCAAAGATGAAG-3′ and the reverse primer 5′-CCAAAAGGAA TTTATTATTTGC-3′. Reaction products were separated on an agarose gel and DNA extracted from a band of the expected size and subcloned into pVAX. The PCR product was sequenced to confirm accurate amplification of the Vcsa1 gene.
For quantitative PCR, the total RNA was extracted from frozen tissue with TRIzol according to the manufacturer’s instruction. Briefly, ≈50 mg tissue was added to 1 mL of TRIzol reagent and homogenized using a polytron homogenizer (Brinkman, Westbury, NY, USA) for 30 s. The homogenized tissues were incubated for 5 min at room temperature followed by adding 200 μL of chloroform. After mixing, the aqueous phases were separated by centrifugation (12 000 g for 15 min) at 4 °C and then were transferred to a clean tube. The RNA was precipitated from the aqueous phase by addition of isopropyl alcohol and pelleted by centrifugation at 12 000 g for 15 min at 4 °C, washed once with 75% ethanol, and again pelleted at 12 000 g for 15 min The ethanol was aspirated and the RNA pellet was dissolved in sterile water; 1 μg of total RNA was reverse-transcribed to first-strand cDNA primed with Oligo(dT) using the Superscript (Invitrogen) First-Strand Synthesis System for real-time PCR. RNA was denatured for 5 min at 65 °C and immediately cooled on ice. Then RNA was combined with the Superscript II RT, 40 units of RNaseOUT recombinant ribonuclease inhibitor, and RT reaction buffer. cDNA was synthesised for 50 min at 42 °C; RT products then were amplified using Sybr Green 2 × PCR Master Mix (PE Applied Biosystems, Warrington, UK). Real-time quantitative PCR analysis was performed using the 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The primers for Vcsa1 were: forward primer, 5′-GAGGGTGTCAGAGGCCC-3′; reverse primer, 5′-GAGCAGTTAGCTGCCACTGATA-3′ (nucleotides 147–163 and 364–384, respectively). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward primer, 5′-GCCGCCTGCTTCACCACCTTCT-3′; reverse primer, 5′-GCATGGCCTTCCGTGTTCCTACC-3′) was used as an endogenous control. The PCR reactions for all samples were performed in 96-well plates, with 2 μL cDNA, 100 nM of each primer, and 12.5 μL of Sybr Green in a 25-μL reaction volume. The cycling conditions were: activation of Sybr Green DNA polymerase at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 15 s, annealing/ extension at 60 °C for 1 min Results from real-time PCR were presented as threshold cycles normalized to that of the housekeeping gene GAPDH, as previously described [16]. The expression of transcripts was analysed by the comparative crossing threshold (Ct) method (also known as the 2− (Δ Δ)Ct method [16]). The relative quantified value for each target gene in diabetic rats compared with control rats is expressed as 2− (Ct–Cc), where Ct and Cc are the mean threshold cycle differences after normalizing to GAPDH. This method was applicable because the efficiency of the primers in generating Vcsa1 product was close to that of the GAPDH gene.
RESULTS
Cavernosometry was used to evaluate erectile capacity in rats after 1 week or 2 months of STZ-induced diabetes compared with control rats (no diabetes). The mean amplitude of the ICP response was examined at 0.75 and 4 mA of stimulation and expressed as the mean ICP/BP during 60 s of CN stimulation. After 1 week of diabetes there was no significant difference from control rats in the basal ICP/BP or after 0.75 and 4 mA stimulation (Table 1). The ICP/BP values were in the range associated with normal erectile capacity.
However, after 2 months of diabetes, although there was no significant difference in the basal ICP/BP, there was a significant decrease in erectile function between the control and diabetic rats at 0.75 and 4 mA stimulation. The decrease in ICP/BP is consistent with the development of ED in the 2 month diabetic rat, as previously reported [15].
Using the ICP/BP data as criteria, we designed experiments to correlate the changes in the expression of Vcsa1 with the onset of diabetes and development of ED. At 1 week, the rats have diabetes but no significant erectile impairment, whereas after 2 months there are obvious alterations in erectile capacity. Quantitative PCR was used to measure Vcsa1 expression in control, 1-week and 2-month diabetic rats. In addition to corporal tissue, we assessed Vcsa1 gene expression in the bladder, urethra and ureter. The Ct values of the samples were compared with bladder from control rats, which we used as the calibrator tissue.
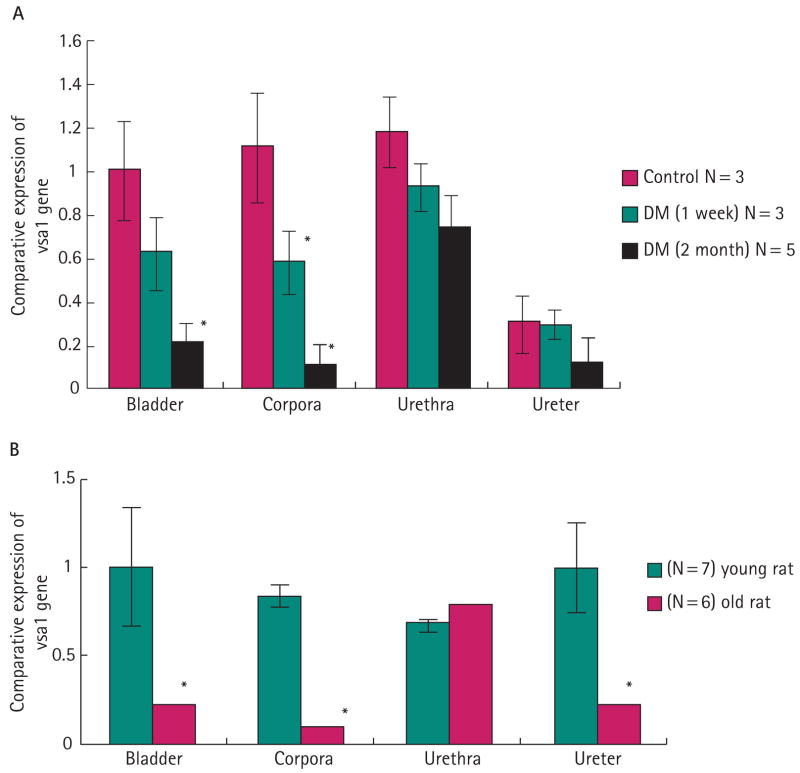
After 1 week of diabetes, there was no significant change in the expression of Vcsa1 compared with control rats in the bladder, urethra or ureter (Fig. 1A). However, in the corpora, Vcsa1 expression was decreased by half. After 2 months of diabetes, there was an even greater decrease in the corpora, with a 10-fold decrease in Vcsa1 expression, and a significant decrease in the bladder of ≈5-fold. These results show a correlation between decreased expression of Vcsa1 in the corpora and ICP/BP measurements.
FIG. 1.
Expression of Vcsa1 transcripts normalized to GAPDH were analysed using the Ct method. Vcsa1 gene expression was determined in corpora, bladder, urethra, and ureter in: A, control (no diabetes), DM (STZ-induced diabetic) 1-week, or DM 2-month rats. The 2-month control bladder tissue was used as the calibrator tissue (set as 1). *Significantly different expression of Vcsa1 vs control value for a particular tissue (P < 0.5). B, in young and old rats. The bladder tissue of young rats was used as the calibrator tissue (set as 1). *Significantly different expression of Vcsa1 compared to the value for tissue from the young rat (P < 0.5). N, number of rats. Each quantitative PCR measurement was duplicated for each tissue. The bars represent the mean comparative expression of the gene, and the error bars the sd.
We also used a rodent model of age-related ED to evaluate further the relationship between alterations in the ICP/BP values and the expression of Vcsa1 in young and old rats (retired breeders). Again, we examined the ICP/BP response at 0.75 and 4 mA. Although the basal ICP/BP were similar in young and old rats (Table 1), the response to CN stimulation was significantly lower in old rats.
In the old rats (which had decreased ICP/BP and ED), again there was a significant decrease in Vcsa1 expression in the corpora, of ≈11-fold (Fig. 1B). Interestingly, expression was also reduced in the bladder and ureter of the old rats by ≈5-fold. There was little effect of ageing on Vcsa1 expression in the urethra.
To confirm the previous observations of User et al. [1], we bilaterally ligated the CNs of four rats and, 9 days after ligation, analysed Vcsa1 expression in the corporal tissue using real-time PCR and compared this with the controls. Before surgery, all four rats had visible erections when stimulated at 6 mA. However, 9 days after surgery, the rats had no visible erection when stimulated at 6 mA. Using real-time PCR there was a 10-fold decrease (±0.8) in Vcsa1 expression in the corpora of CN-ligated rats compared with the controls (four).
Overall, the above experiments show that in three rat models of ED (diabetic, age-related, and neurogenic) the Vcsa1 gene in corporal tissue is down-regulated. To test the hypothesis that Vcsa1 plays a direct role in modulating erectile capacity, we used gene transfer experiments whereby we injected intracorporally in old rats a plasmid (pVAX-Vcsa1), where expression is driven from the cytomegalovirus (CMV) promoter. As positive controls, old rats were injected with pVAX-hSlo [12, 13], which ameliorates the age-related decline of erectile capacity in this rodent model. Finally, negative controls were run in parallel, where rats were injected with the backbone plasmid (pVAX). At 1 week after injection, the ICP/BP was compared before corporal tissue excision, and the rats were assessed by light microscopic examination (Table 2).
TABLE 2.
Measurement of erectile function (ICP/BP) in old rats (9–10 months) after intracorporal injection of pVAX, pVAX-hSlo, or pVAX-Vcsa1
| Mean (sd) ICP/BP
|
||||
|---|---|---|---|---|
| Treatment (dose, μg) | N | Basal | CN stimulation 0.75 mA | CN stimulation 4 mA |
| pVAX (100) | 8 | 0.063 (0.020) | 0.22 (0.12) | 0.33 (0.18) |
| pVAX-hSlo (100) | 6 | 0.085 (0.030) | 0.69 (0.033)* | 0.75 (0.06)* |
| pVAX-Vcsa1 (80) | 4 | 0.148 (0.071)* | 0.35 (0.21)* | 0.41 (0.18)* |
| pVAX-Vcsa1 (25) | 3 | 0.097 (0.029) | 0.54 (0.21)* | 0.62 (0.13)* |
| pVAX-Vcsa1 (5) | 3 | 0.073 (0.023) | 0.43 (0.11)* | 0.64 (0.046)* |
The rats weighed >500 g.
P < 0.5 vs control rats (pVAX), one-way anova.
In rats treated with 80 μg pVAX-Vcsa1, there was a significant increase in basal ICP/BP compared with all other groups. After stimulation, there was a slight improvement in ICP/BP compared with the negative control, which did not result in a visible erection, except in one of the four rats that, after stimulation, had priapism. Visual postmortem examination of the rats injected with pVAX-Vcsa1 showed evidence of vascular congestion of the corporal tissue, suggesting that the rats had priapism. This was confirmed by microscopic examination of rats injected with 80 μg pVAX-Vcsa1 (Fig. 2). There was trabecular interstitial oedema, blood clot formation within the cavernosa, and destruction of the endothelial lining, suggesting that priapism had been severe in the week between injection of the plasmid and termination of the experiment. Neither negative controls nor rats injected with the same dose of pVAX-hSlo showed any signs of priapism.
FIG. 2.
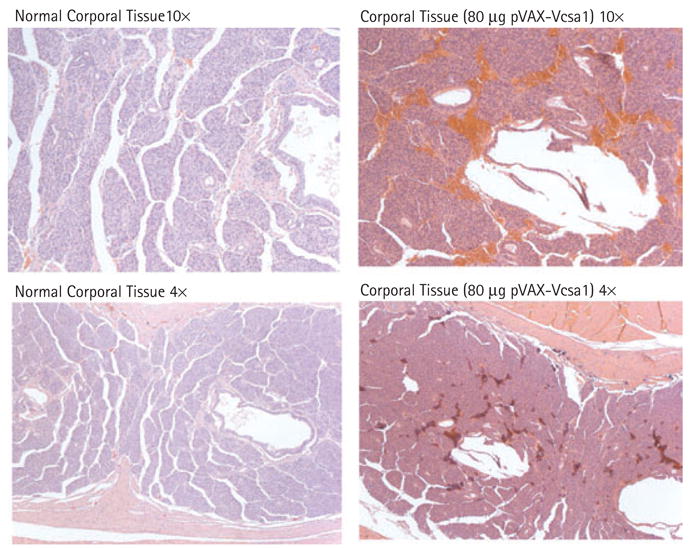
Histological analysis of corporal tissue sections 1 week after intracorporal injection with 80 μg pVAX-Vcsa1 compared with untreated control tissue. Magnification either × 4 or × 10.
At lower doses of pVAX-Vcsa1 (5 and 25 μg) there was a significant improvement in erectile response, as indicated by the ICP/BP compared with control rats (treated with the empty vector pVAX; Table 2). At the highest intensity of stimulation (4 mA), there was no significant difference in ICP/BP after gene transfer of pVAX-Vcsa1 or pVAX-hSlo, which was reported to restore erectile function in old rats and diabetic rats, and is undergoing clinical phase I trials for treating ED. However, after CN stimulation, the time to return to basal ICP/BP in rats treated with 5 and 25 μg pVAX-Vcsa1 was about twice that in rats treated with pVAX-hSlo. These results suggest that intracorporal injection with pVAX-Vcsa1 results in a disturbance in the normal outflow of blood from the penis, which at low doses may improve erectile function, but at the higher dose of 80 μg pVAX-Vcsa1 can result in priapism.
DISCUSSION
The present study for the first time identifies a gene, Vcsa1, that has altered expression in three models of ED and therefore might be a marker for organic ED. In addition, we show a physiological effect of the gene on erectile function. Gene transfer of a plasmid expressing the gene (pVAX-Vcsa1) seems to cause increased blood flow into the corpora in lower doses resulting in increased ICP/BP in old rats after CN stimulation, but causing priapism at higher doses.
Vcsa1 was previously reported to be down-regulated in another model of ED, ligation of the CN that simulates nerve damage (neurogenic ED) [1]. In the present study, we confirmed that observation, and showed that the Vcsa1 gene was also down-regulated in two other models of ED, a rat model for ageing and STZ-induced diabetes. Although ED has overlapping pathophysiology, this is the first report of a common molecular change in three models of ED. We therefore suggest that it might be a useful molecular marker for organic ED. It is possible that in this role it could act as a very early marker of ED, as, after 1 week of diabetes, when there is a small but not significant decrease in ICP/BP compared to controls (Table 1), the expression of the Vcsa1 transcript in the corpora is decreased by about a half (Fig. 1A). Because the regulation of Vcsa1 is under adrenergic control [17], it is possible that the down-regulation of Vcsa1 in ED associated with ageing and diabetes is a result of CN neuropathy that occurs with both diabetes and ageing [18].
The exact function of the Vcsa1 gene has not been determined. However, it is thought to act as a precursor hormone, and a peptide product of the gene was shown to modulate the male rat’s sexual behaviour [3]. In the study of Messaoudi et al. [3] in rats treated with sialorphin, there was a significant stimulatory effect on the frequency of intromissions before ejaculation and on the propensity of males to engage in investigatory behaviour directed to the female during the postejaculatory intervals. That study did not directly address the effect of sialorphin on erectile capacity, and the rats were of an age and health status where erectile capacity would not have shown significant pathological features. In the present study, it was possible to see the physiological effect of the Vcsa1 gene only in rats that had a reduced erectile function because of their age. In addition, our experiments support the role of Vcsa1 as a pro-hormone. Gene transfer by intracorporal injection results in only a subset of corporal cells taking up the gene and expressing it. Therefore, an effect on the general physiology of the corpora suggests that the product of the gene affects more than a subset of genes.
The rat Vcsa1 gene shows 38% identity with a human homologue, submaxillary gland androgen-regulated protein 3 homologue A precursor, SMR3A [19]. The homology suggests that any role for Vcsa1 found in the rat might be taken by SMR3A in humans.
Overall, the present study suggests that Vcsa1 will be a useful marker for organic ED. It also shows that Vcsa1 is somehow involved in the regulation of blood circulation in the corpora. Because regulation of blood flow in corporal tissue is a key regulator of erectile function, this gene may also represent a novel therapeutic target for treating ED.
Acknowledgments
This work was supported by grants P01-DK060037 and K01-DK67270 (awarded to K.P. Davies) from the National Institutes of Health, National Institute of Digestive, Diabetes and Kidney Diseases.
Source of funding: NIH, NIDDK grants P01-DK060037 and K01-DK67270 (awarded to Kelvin Davies).
Abbreviations
- Vcsa1
variable coding sequence protein A1
- SMR-1
submandibular rat 1 protein
- ED
erectile dysfunction
- STZ
streptozotocin
- CN
cavernosal nerve
- ICP/BP
intracorporal/blood pressure
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- CMV
cytomegalovirus
- Ct
comparative crossing threshold
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.User HM, Zelner DJ, McKenna KE, McVary KT. Microarray analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;170:298–301. doi: 10.1097/01.ju.0000060882.75475.5a. [DOI] [PubMed] [Google Scholar]
- 2.Rougeot C, Rosinski-Chupin I, Njamkepo E, Rougeon F. Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur J Biochem. 1994;219:765–73. doi: 10.1111/j.1432-1033.1994.tb18556.x. [DOI] [PubMed] [Google Scholar]
- 3.Messaoudi M, Desor D, Nejdi A, Rougeot C. The endogenous androgen-regulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684–91. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Rougeot C, Messaoudi M, Hermitte V, et al. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci USA. 2003;100:8549–54. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rougeot C, Vienet R, Cardona A, Le Doledec L, Grognet JM, Rougeon F. Targets for SMR1-pentapeptide suggest a link between the circulating peptide and mineral transport. Am J Physiol. 1997;273:R1309–20. doi: 10.1152/ajpregu.1997.273.4.R1309. [DOI] [PubMed] [Google Scholar]
- 6.Rosinski-Chupin I, Rougeot C, Courty Y, Rougeon F. Localization of mRNAs of two androgen-dependent proteins, SMR1 and SMR2, by in situ hybridization reveals sexual differences in acinar cells of rat submandibular gland. J Histochem Cytochem. 1993;41:1645–9. doi: 10.1177/41.11.8409372. [DOI] [PubMed] [Google Scholar]
- 7.Lizza EF, Rosen RC. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res. 1999;11:141–3. doi: 10.1038/sj.ijir.3900396. [DOI] [PubMed] [Google Scholar]
- 8.Korenman SG. Epidemiology of erectile dysfunction. Endocrine. 2004;23:87–91. doi: 10.1385/ENDO:23:2-3:087. [DOI] [PubMed] [Google Scholar]
- 9.Shabsigh R, Perelman MA, Lockhart DC, Lue TF, Broderick GA. Health issues of men: prevalence and correlates of erectile dysfunction. J Urol. 2005;174:662–7. doi: 10.1097/01.ju.0000165389.73148.d1. [DOI] [PubMed] [Google Scholar]
- 10.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170:S6–14. doi: 10.1097/01.ju.0000075362.08363.a4. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan CJ, Teal TH, Luttrell IP, Tran KB, Peters MA, Wessells H. Microarray analysis reveals novel gene expression changes associated with erectile dysfunction in diabetic rats. Physiol Genomics. 2005;23:192–205. doi: 10.1152/physiolgenomics.00112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christ GJ, Rehman J, Day N, et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998;275:H600–8. doi: 10.1152/ajpheart.1998.275.2.H600. [DOI] [PubMed] [Google Scholar]
- 13.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170:285–90. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- 14.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur Urol. 2005;48:314–8. doi: 10.1016/j.eururo.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Christ GJ, Day N, Santizo C, et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1544–53. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Rosinski-Chupin I, Huaulme JF, Rougeot C, Rougeon F. The transcriptional response to androgens of the rat VCSA1 gene is amplified by both binary and graded mechanisms. Endocrinology. 2001;142:4550–9. doi: 10.1210/endo.142.10.8428. [DOI] [PubMed] [Google Scholar]
- 18.Bleustein CB, Arezzo JC, Eckholdt H, Melman A. The neuropathy of erectile dysfunction. Int J Impot Res. 2002;14:433–9. doi: 10.1038/sj.ijir.3900907. [DOI] [PubMed] [Google Scholar]
- 19.Isemura S, Saitoh E. Nucleotide sequence of gene PBI encoding a protein homologous to salivary proline-rich protein P-B. J Biochem (Tokyo) 1997;121:1025–30. doi: 10.1093/oxfordjournals.jbchem.a021689. [DOI] [PubMed] [Google Scholar]