Abstract
The mechanical properties and density of natural and synthetic extracellular matrices are known to affect cellular processes and regulate tissue formation. In this report, these factors were independently investigated for their role in ovarian follicle development. The matrix composition was controlled through decreasing the solids concentration or the molar mass of the encapsulating biomaterial, alginate. Decreasing matrix stiffness and solids concentration enhanced follicle growth, and coordinated differentiation of the follicle cell types, as evidenced by antral cavity formation, theca cell differentiation, oocyte maturation, and relative hormone production levels. While a stiff environment favored high progesterone and androgen secretion, decreasing alginate stiffness resulted in estrogen production which exceeded progesterone and androgen accumulation. These studies reveal, for the first time, a direct link between the biomechanical environment and follicle function, and suggest a novel non-hormonal mechanism regulating follicle development.
Introduction
The physical properties of the extracellular matrix, such as mechanical stiffness, affect cell behavior in a variety of tissues [1, 2], including those which are not thought to be mechanically challenged [3-7]. Specifically, the physical properties of hydrogels regulate cellular processes such as cell proliferation and growth factor and extracellular matrix production, leading to tissue formation in engineered tissues [8-10]. The relationship between the physical properties and cellular responses has been investigated for a number of cell types; however, the relationship between the physical properties of the matrix and the properties of the tissue are more difficult to investigate in vitro given that many tissues cannot be grown due to limits on vascularization. Ovarian follicle culture systems provide an ideal system in which to correlate the physical properties of a three-dimensional hydrogel matrix to development since follicles are not vascularized, and development can be easily monitored because follicles pass through distinct morphologic and steroidogenic stages as they mature.
The hormonal regulation of folliculogenesis has been widely investigated, yet the role of the physical properties of the follicle microenvironment has not. Understanding the role of the physical environment on follicle development will be useful for the development of biomimetic matrices for the in vitro culture of follicles and other hydrogel-encapsulated cell culture systems. In addition, this understanding may identify mechanical mechanisms underlying ovarian disorders such as polycystic ovarian syndrome, a condition that impacts nearly 10% of all reproductive age women. Ultimately, development of a culture system presenting the proper mechanical and biochemical signals to support human folliculogenesis in vitro may provide a means to preserve reproductive potential for females facing premature infertility due to cancer therapies.
Three dimensional culture systems maintain the three-dimensional structure of mouse follicles in vitro [11, 12], which more faithfully mimics the in vivo environment relative to two-dimensional systems [13]. The ovarian follicle consists of a centrally located oocyte surrounded by one or more layers of somatic granulosa and theca cells, which support follicle development. As follicles develop, the somatic cells surrounding the oocyte proliferate and differentiate, and the oocyte grows in preparation for ovulation and fertilization. To date, most systems designed to support follicle growth in vitro have been two-dimensional [13-15]. In such systems, follicles attach to a two-dimensional culture surface, and somatic cells typically migrate away from the oocyte. This spreading alters the three-dimensional structure of the follicle, thereby disrupting the cell-cell interactions important to the exchange of necessary metabolic precursors from the somatic cells to the egg [16, 17]. Three-dimensional culture systems in our laboratory support follicle survival and growth for 12 days in mouse and up to forty days in non-human primate follicles (unpublished observations). Oocytes contained in encapsulated follicle cultures mature and are of sufficient quality to be fertilized. Live pups born from the in vitro-matured eggs are healthy and fertile [18].
We have employed alginate as a three dimensional matrix for the encapsulation and maturation of ovarian follicles to produce mature fertilizable oocytes [11, 12]. Alginate is a widely used biomaterial in tissue engineering applications [19], and is suitable for follicle culture due to its gentle gelling properties and biochemical characteristics [20-23]. Alginate is produced by brown algae, and is a linear polysaccharide copolymer of β-d-mannuronic acid and α-l-guluronic acid [24, 25]. Alginate gels by ionic crosslinking in the presence of divalent cations, which are not harmful to the encapsulated follicles [12]. The alginate gel forms a mesh-like structure that permits diffusion of hormones and other proteins essential for follicle development. Follicle stimulating hormone (FSH), an essential hormone for in vivo follicle development, is able to diffuse through the alginate, and causes a dose-dependent increase in in vitro follicle growth [26]. While our first reports of follicle cultures demonstrated growth in vitro, encapsulated follicles did not achieve the sizes typically observed in vivo, and few follicles differentiate to form a stratified theca cell layer or fluid-filled antral cavity characteristic of mature follicles [11, 12, 26]. Recent results in our lab have suggested that the physical properties of the matrix may influence follicle development [27].
This report investigates the hypothesis that the matrix stiffness and density may limit expansion of the follicle and consequently oocyte maturation. To test the hypothesis, alginate hydrogels ranging in stiffness were formed via varying the alginate solids concentration, or by treating the polymer with radiation or chemical oxidation [28-30]. Matrices were developed to represent conditions with constant density with varied stiffness, as well as constant stiffness with varied density. The mechanical properties of each condition were characterized by measurement of the shear elastic modulus. Using a mouse model, two-layered and multilayered secondary follicles were isolated and encapsulated in each hydrogel condition, and growth, morphology, and hormone production were assessed. At the end of culture, the quality of the oocytes was assessed based upon their ability to resume meiosis. Identifying the mechanical properties and solids concentration of the hydrogel that maximize follicle development represents a new idea in our understanding of ovarian follicle development, and is a critical step toward developing a system that will enable follicle growth following tissue banking.
Materials and Methods
Animals and materials
Male CBA and female C57BL/6 mice (Harlan, Indianapolis, IN) were housed as breeding pairs in a temperature- and light-controlled environment and provided with food and water ad libidum. Animals were fed phytoestrogen-free Teklad Global irradiated 2919 chow. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and protocols were approved by the IACUC at Northwestern University. Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and media formulations from Invitrogen (Carlsbad, CA).
Alginate preparation
Sodium alginate (55-65% guluronic acid) was generously provided by FMC BioPolymers (Philadelphia, PA). Radiation-treated alginate was placed in a Cesium-137 irradiator (M38-1 Gammator, Radiation Machinery Corp., Parsippany, NJ) and exposed to doses of gamma radiation ranging from 0.25 to 5 Mrad. Prior to encapsulation and shear modulus measurement, the 5 Mrad-treated alginate was blended with 50% untreated alginate so that the resulting gel maintains sufficient integrity for follicle encapsulation. Oxidized alginate was treated to a theoretical extent ranging from 0.25-5% (i.e. 0.25-5% of alginate monomers oxidized) with sodium periodate as previously described [29] and dialyzed four days to remove the remaining reactants. The 1% oxidized alginate was used for all follicle encapsulations. Aliquots of charcoal-stripped and sterilized alginate were reconstituted overnight with sterile 1X phosphate buffered saline to 0.7%, 1.5%, or 3% (w/v) concentrations.
Alginate characterization
Alginate molar mass was measured by gel permeation chromatography (GPC) to characterize the extent of polymer degradation following radiation and oxidation. Briefly, each alginate solid was dissolved in water at a concentration of 2 mg/ml, and injected into a series of three size-exclusion columns (Shodex SB-806 HQ, SB-804 HQ, and SB-802.5 HQ, Showa Denko, Kawasaki, Japan). In this tandem GPC-MALLS mode, the effluent from the GPC system flows directly into a DAWN DSP Laser Photometer and Optilab DSP Interferometric Refractometer connected in series (both Wyatt Technology, Santa Barbara, CA). The flow rate was 0.3 ml/min and the mobile phase consisted of 100 mM NaCl, 50 mM NaH2PO4, and 200 ppm NaN3. The tandem GPC-MALLS data were processed using ASTRA software (Wyatt Technology, Santa Barbara, CA) to determine weight average molar mass (Mw), radius of gyration (Rg), and polydispersity index (PDI). Three separate measurements were collected for each alginate condition and the results were averaged.
Alginate shear elastic modulus was measured at 25°C using a Paar Physica MCR Rheometer (Anton Paar, Graz, Austria) using a parallel plate geometry (diameter of 50 mm, gap of 1 mm) and Paar Physica US200 software (Anton Paar, Graz, Austria). Alginate and a 40% (w/v) CaSO4 slurry were quickly blended (40 μl CaSO4 slurry/ml alginate) and extruded onto the lower plate of the rheometer. The upper plate was immediately lowered, and the alginate crosslinked between the plates for two hours. Storage moduli were determined by oscillatory shear experiments performed with a strain amplitude of 1% and oscillation frequency of 10 rad/s, which is within the linear viscoelasticity region, as verified by frequency and strain sweeps following each measurement.
Follicle isolation, encapsulation, and culture
Two-layered (100-130 μm) and multilayered (150-180 μm) secondary follicles were mechanically isolated from the ovaries of 12- and 16-day old C57BL/6×CBA F1 female mice, respectively, as previously described [12]. Follicles were then transferred into beads of alginate solution on a polypropylene mesh (0.1 mm opening) and immersed in a calcium-containing solution (50 mM CaCl2, 140 mM NaCl) for two minutes to crosslink the alginate solution as previously described [12, 26]]. The beads were then rinsed and transferred to growth medium composed of αMEM supplemented with 10 mIU/ml recombinant human follicle stimulating hormone (Organon, Roseland, NJ), 3 mg/ml bovine serum albumin, 1 mg/ml bovine fetuin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium. Beads were placed in individual wells of a 96-well plate containing 100 μl growth medium, and cultured for eight to twelve days. Half the medium (50 μl) was exchanged every two days. The conditioned media was stored at -80°C for hormonal analysis. Follicle images were collected using a Leica DM IL light microscope (Leica, Wetzlar, Germany) equipped with phase objectives and a heated stage, a Spot Insight 2 Megapixel Color Mosaic camera, and Spot software (Spot Diagnostic Instruments, Sterling Heights, MI). Follicle diameters were measured using ImageJ software (National Institutes of Health, USA).
Oocyte meiotic competence
Oocyte meiotic competence was assessed by maturation after twelve days of culture for two-layered secondary follicles and after eight days of culture for multilayered secondary follicles. Follicles were removed from beads by incubating the beads in a 10 IU/ml solution of alginate lyase in pre-equilibrated in alpha minimum essential medium (αMEM) at 37°C for one hour, which enzymatically degrades the alginate bead. Follicles were then transferred to αMEM containing 0.25 pg/ml epidermal growth factor and 45 mIU/ml human chorionic gonadotropin and incubated at 37°C for 14-16 hours. Oocytes were removed from follicles, and images collected by Hoffman Modulation Contrast microscopy using a Leica DM IL microscope. Oocyte state was assessed from the images, and characterized as DG (degenerated), GV (intact germinal vesicle), or GVBD (germinal vesicle breakdown) based on the presence or absence of a germinal vesicle and polar body.
Hormone assays
Androstenedione, 17β-estradiol, and progesterone were measured using commercially available radioimmunoassay kits (androstenedione and 17β-estradiol, Diagnostic Systems Laboratories, Inc., Webster, TX; progesterone, Diagnostic Products Corporation, Los Angeles, CA). The sensitivities for the androstenedione, estradiol, and progesterone assays are 0.1 ng/ml, 10 pg/ml, and 0.1 ng/ml, respectively. Assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. To obtain sufficient media for each assay, media collected from follicles in identical alginate conditions was pooled for each time point (12-20 samples pooled per measurement).
Statistics
Statistical calculations were performed using JMP 4.0.4 software (SAS Institute, Cary, NC). Statistical significance for follicle size measurements and steroid levels was analyzed using a two-way ANOVA with repeated measures, or one-way ANOVA followed by Tukey-HSD for single time points. X2 analysis was used to analyze categorical data.
Results
Alginate characterization
This report investigates ovarian follicle maturation as a function of the physical properties of the hydrogel, thus, the molar mass and shear modulus of various alginate formulations were measured. The molar mass of alginate decreased with increasing radiation and oxidation treatment as shown in Table 1. The oxidation and radiation conditions selected (1% oxidation and 5 Mrad, respectively) decreased the molecular mass by factors of 1.4 and 5.0, respectively. The polydispersity of the polymer increased with both oxidation and radiation indicating a broader range of polymer molar masses present.
Table 1.
Alginate polymer and hydrogel characterization: Shear elastic modulus data represented as average±SEM from three or more independent measurements
| alginate | Mw (kDa) | PDI | G’(Pa) |
|---|---|---|---|
| 3% | 418 | 2.07 | 3010±84a |
| 1.5% | 418 | 2.07 | 1300±129b |
| 0.7% | 418 | 2.07 | 203±13c |
| ox | 293 | 2.38 | 629±62c |
| irr | 83 | 2.00 | 384±77c |
Mw=weight average molar mass, G’=shear elastic modulus, kDa=kilodaltons, PDI=polydispersity, Pa=pascals. In this and other tables and figures, 3% = 3% untreated, 1.5% = 1.5% untreated, 0.7% = 0.7% untreated, ox = oxidized (1.5% w/v), and irr = irradiated (1.5% w/v). Significant differences between conditions are indicated by different letters (p<0.05)
Multiple alginate formulations were prepared and the shear modulus was measured. Formulations consisted of the following: 1) 3% alginate 2) 1.5% alginate, 3) 0.7% alginate, 4) 1.5% oxidized alginate, and 5) 1.5% irradiated alginate. The shear elastic modulus (G’) was decreased by decreasing alginate solids concentration as well as by oxidation and radiation treatment (Table 1). The oxidized, irradiated, and 0.7% alginate conditions had significantly lower moduli than the 1.5% and 3% alginate conditions, and do not differ significantly from one another. 3% alginate has a shear elastic modulus significantly higher than all other alginate conditions, while 1.5% alginate has an intermediate shear elastic modulus. Oxidized alginate can degrade over time via hydrolysis [29], however in our system, alginate elastic modulus measurements varied minimally over 12 days (data not shown). This observation can likely be attributed to the extensive dialysis following oxidation that hydrolyzes the polymer prior to follicle encapsulation. For subsequent studies investigating follicle growth, note that conditions 3-5 have similar mechanical properties for comparison of matrix density, and conditions 2, 4, and 5 have the same solids concentration for comparison of varying mechanics.
Two-layered and multilayered secondary follicle encapsulation and growth
Follicle growth was investigated in hydrogels with varied stiffness. Media conditions were held constant. A minimum of 130 two-layered secondary follicles were encapsulated for each alginate condition and cultured for 12 days. At the end of culture, 15-42% of two-layered secondary follicles remained viable. Follicles cultured in hydrogels with a decreased stiffness yielded greater increases in follicle diameter (Figure 1a-b). Two-layered follicles cultured in high-modulus 3% alginate increased 16.6% in diameter during 12 days of culture, which is significantly less than the percent increases in diameter of 97.8, 106.4, and 107.4 in the case of 0.7% alginate, oxidized alginate, and irradiated alginate, respectively (Figure 1b). Follicles cultured in the mid-range shear modulus alginate (1.5% alginate) increased 79.0% in diameter, which is a significant decrease relative to the growth in irradiated and oxidized alginate. Varying the method of alginate treatment to decrease shear modulus (decreasing alginate concentration, oxidation, and irradiation) did not significantly affect growth of follicles.
Figure 1.

Two-layered secondary follicle growth: (A) Growth over a 12 day culture period. (B) Percent increase in follicle diameter at day 12 (relative to day 0) of culture (p<0.05). (C-F) A representative follicle at day 0 of culture (C), and at day 12 of culture in 3% (D), 1.5% (E), and irradiated (F) alginate. Oo = oocyte, scale bar = 50 μm. Data represented as average ± SEM from two or more independent experiments for each alginate condition.
Multilayered secondary follicles demonstrated a similar trend for follicle growth in hydrogels. A minimum of 60 multilayered secondary follicles were encapsulated in alginate of each condition and 83-91% of the follicles remained viable following eight days of culture. Similarly to the two-layered follicles, all surviving multilayered secondary follicles in alginate grew during the culture period, and hydrogels with a decreased stiffness yielded greater increases in follicle diameter (Figure 2a,b). Follicles cultured in 3% alginate averaged a 24.7% increase in follicle diameter, while those cultured in 1.5% alginate increased 92.1% in diameter (Figure 2b). Follicles in the lowest shear elastic moduli alginate conditions demonstrated significantly greater increases in follicle diameter relative to 3% and 1.5% alginate (Figure 2a,b). Follicles in 0.7% alginate, oxidized alginate, and irradiated alginate increased in diameter 131.5%, 147.2%, and 139.8%, respectively. Varying the method of alginate treatment to decrease shear modulus did not significantly affect the growth of multilayered secondary follicles.
Figure 2.
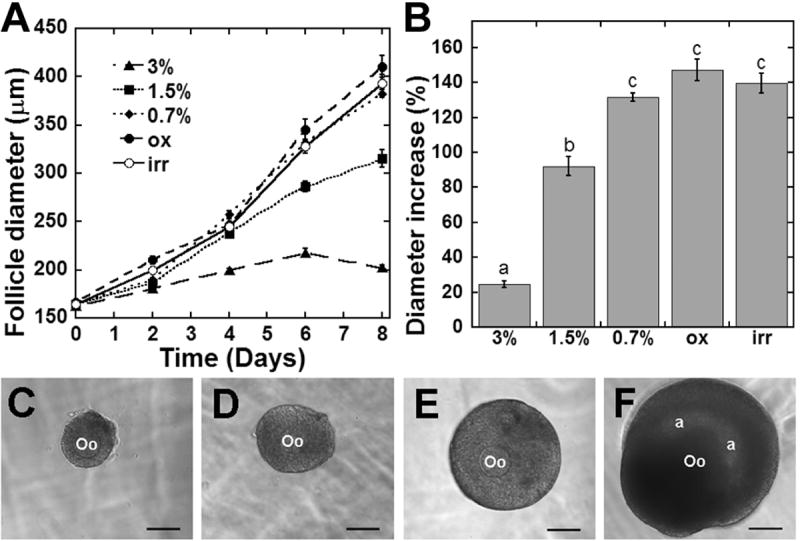
Multilayered secondary follicle growth: (A) Growth in alginate over an 8-day culture period. (B) Percent increase in follicle diameter at day 8 (relative to day 0) of culture. (C-F) A representative follicle at day 0 of culture (C), and at day 8 of culture in 3% (D), 1.5% (E), and 0.7% (F) alginate. Oo = oocyte, a = antral cavity, scale bar = 100 μm. Significant differences between alginate conditions are indicated by different letters (p<0.05). Data represented as average ± SEM from two or more independent experiments for each alginate condition.
Follicle morphology and cell differentiation
The formation of an antrum, a fluid-filled central cavity that develops during follicle development in vivo, was subsequently characterized for hydrogels of varying mechanics and density. Follicles encapsulated in 1.5% alginate did not form an antrum [26], and recent reports suggest that the matrix mechanics may impact antrum formation [27]. Antrum formation was observed in the alginate-encapsulated follicles (Figure 2f), and the percentage of both two-layered and multilayered secondary follicles forming antra increased with decreasing shear elastic modulus of the encapsulating alginate (Figure 3). A larger percentage of multilayered follicles than two-layered secondary follicles were able to form antra in all alginate conditions. In both follicle classes, the rate of antrum formation was significantly higher in the 0.7% alginate condition than in other conditions (36.8% of two-layered secondary follicles and 80.6% of multilayered secondary follicles). Irradiated and oxidized alginate also supported increased rates of antrum formation relative to 3% and 1.5% alginate, although this increase is only significant in the case of multilayered secondary follicles.
Figure 3.

Antral cavity formation: Percent of (A) two-layered and (B) multilayered secondary follicles that form an antral cavity by the end of culture. (p<0.05)
Theca formation was also enhanced in low-stiffness alginate. Both two-layered and multilayered secondary follicles formed stratified theca layers in low-stiffness conditions (Figure 4). A theca layer was observed in 16.4% of two-layered secondary follicles cultured in irradiated alginate and 10.7% of multilayered secondary follicles cultured in 0.7% alginate. The theca layer was most often observed surrounding relatively small follicles, perhaps indicating that the theca is present and proliferating in more follicles than observed by visual inspection, but is not discernable because the theca cells are distributed over an increased surface area, thereby decreasing the thickness of the layer.
Figure 4.

Theca layer formation. After 12 days of culture, a stratified theca layer surrounds a follicle encapsulated as a two-layered secondary follicle in irradiated alginate. Oo = oocyte, gc = granulosa cells, tc = theca cells, scale bar = 50 μm.
Steroid secretion
Follicular cells secrete ovarian hormones during normal development, and therefore measurements of estradiol, androstenedione, and progesterone were performed on media collected from cultured follicles. While significant differences in steroid production levels were not detected in two-layered secondary follicles, measurements over the culture period demonstrate that follicles in 0.7% alginate have enhanced steroid production as early as day six of culture. Multilayered secondary follicles secreted significantly more estradiol and androstenedione with decreased alginate stiffness (Figure 5). Measurements at earlier time points were below the detectable limits of the assay. In both follicle size classes, estradiol production was highest in 0.7% alginate, which correlates with the enhanced antrum formation in this low solids concentration alginate condition. Estradiol and androstenedione are produced primarily by granulosa cells and theca cells, respectively, in vivo during development, and production of these steroids in vitro confirms that follicular cells are able to carry out their steroidogenic roles in vitro. Progesterone levels did not differ significantly between alginate conditions in two-layered or multi-layered secondary follicles. Appropriate steroid biosynthesis is reflected in the ratio of secreted estradiol, androstenedione, and progesterone (Table 2). The ratio of progesterone and/or androstenedione to estradiol decreases with decreasing alginate stiffness in the immature follicles. Androgen accumulation is not as pronounced in the multilayered follicle but progesterone is still elevated. Together with the growth and survival data, the steroid profiles suggest that the permissive environment that supports follicle development is one that is less stiff.
Figure 5.
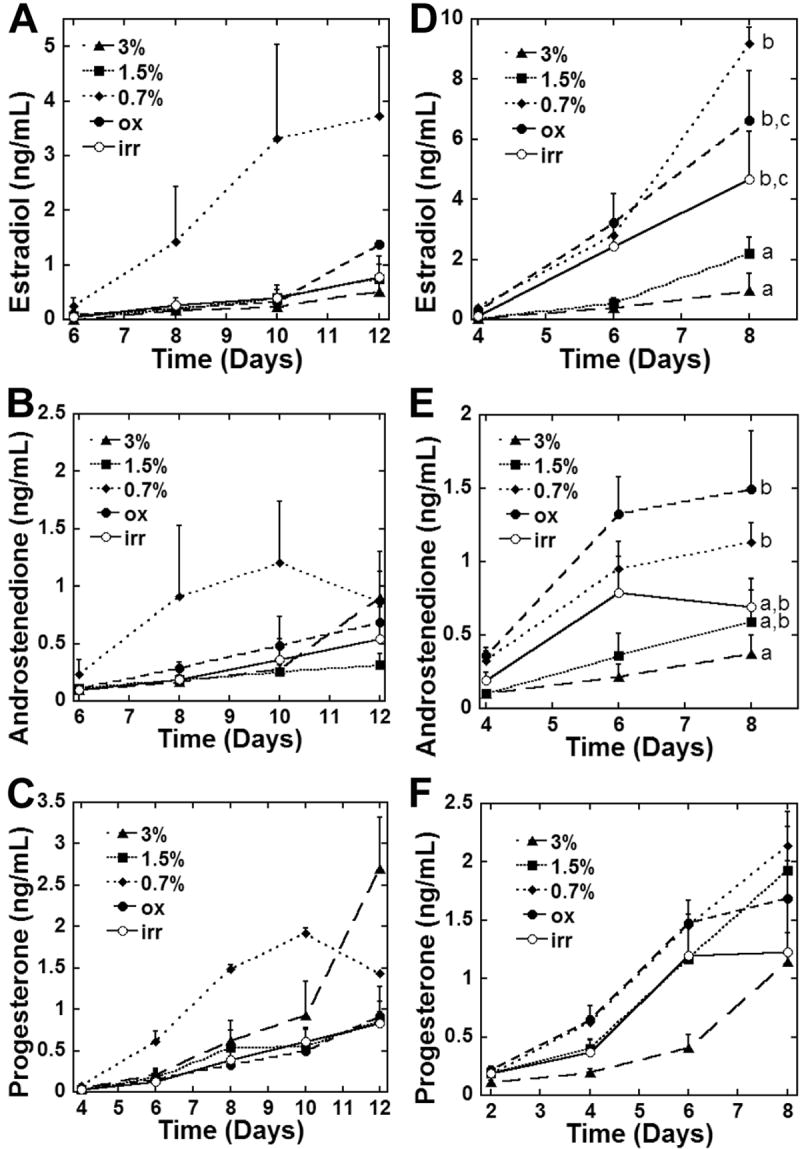
Steroid production by two-layered and multilayered secondary follicles. Estradiol, androstenedione, and progesterone concentrations in culture media during culture and at the final day of culture for two-layered (A-C) and multilayered (D-F) secondary follicles. Significant differences between alginate conditions are indicated by different letters (p<0.05). Data represented as average ± SEM from two or more independent experiments for each alginate condition.
Table 2.
Ratios of estradiol concentration to androstenedione and progesterone concentrations at end of culture
| two-layered | multilayered | |||||
|---|---|---|---|---|---|---|
| alginate | E | A | P | E | A | P |
| 3% | 1 | 1.78 | 5.30 | 1 | 0.38 | 1.18 |
| 1.5% | 1 | 0.42 | 1.16 | 1 | 0.27 | 0.87 |
| 0.7% | 1 | 0.23 | 0.38 | 1 | 0.12 | 0.23 |
| ox | 1 | 0.50 | 0.67 | 1 | 0.22 | 0.25 |
| irr | 1 | 0.69 | 1.08 | 1 | 0.15 | 0.26 |
E=estradiol, A=androstenedione, P=progesterone
Oocyte quality
The quality of oocytes retrieved from follicles cultured within alginate hydrogels was measured by their ability to resume meiosis. Oocytes isolated from follicles cultured in 3% alginate demonstrated the lowest rate of meiotic resumption: 31.3% of two-layered secondary follicles and 46.4% of multilayered secondary follicles (Figure 6). Increased percentages of oocytes able to resume meiosis, as indicated by germinal vesicle breakdown (GVBD), were observed in the lower shear moduli alginate conditions. Irradiated alginate culture yielded the highest percentage of GVBD oocytes from two-layered secondary follicles (65.7%), while 0.7% alginate yielded the highest percentage of GVBD oocytes from multilayered secondary follicles (90.8%).
Figure 6.
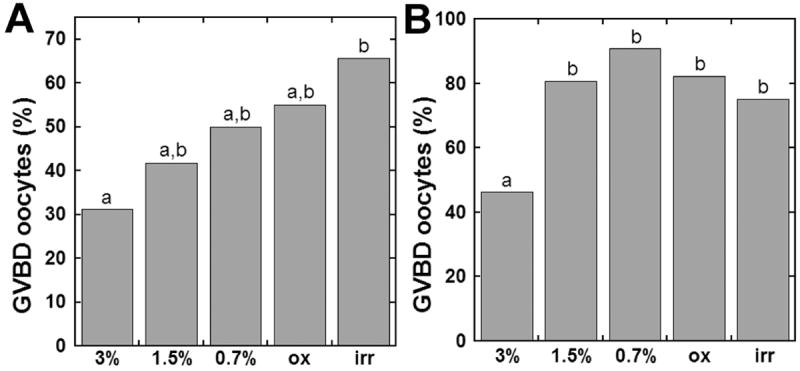
Oocyte quality: Oocytes collected from (A) two-layered and (B) multilayered secondary follicle cultures classified as GVBD following hCG treatment. (p<0.05)
Discussion
We hypothesized that the stiffness and density of the alginate matrix influences the growth and differentiation of multiple cellular compartments within the follicle. Three methods were employed to control the stiffness of alginate: irradiation, oxidation, and variation of solids concentration. The resulting shear elastic moduli (G’) represent a range of three significantly different conditions: high G’ (3% alginate), intermediate G’ (1.5% alginate), and low G’ (0.7%, oxidized, and irradiated alginate). In these matrices, follicle growth increased with decreasing alginate stiffness. Follicles cultured in low G’ alginate also had higher rates of antrum and theca layer formation than follicles cultured in more stiff hydrogels. Additionally, androgen and progesterone were converted to estrogen in less stiff conditions, whereas androgen or progesterone exceeded estradiol levels in the more stiff conditions. Most importantly, oocytes cultured in low G’ alginate were of higher quality, as measured by their ability to resume meiosis, than oocytes cultured in relatively stiff alginate conditions. Taken together, these results indicate that the physical properties of the follicle environment influence cellular processes and the coordination between the cellular compartments necessary for successful maturation. This mechanical regulation of follicle development is new to follicle biology, as endocrine hormones have been widely characterized as the primary factors regulating development. In addition, these findings emphasize the broad impact that the physical properties of a biomaterial may have on engineered tissues, particularly for tissues that are not traditionally thought to be mechanically stimulated.
The role of matrix stiffness and solids concentration were isolated by manipulating the alginate molecular weight and composition of the hydrogels.. A previous report has shown that decreasing the solids concentration leads to enhancement in follicle growth [27]; however, decreasing the percentage of alginate in solution both decreases shear elastic modulus, and may also facilitate diffusion of molecules through the alginate. To examine this question more thoroughly, the stiffness of the matrix was decreased by varying the molar mass of the polymer while maintaining a constant solids concentration. Preparations of untreated, oxidized, and irradiated alginate had similar solids concentration (1.5% w/v); however, the shear modulus ranged from 200 Pa to 1300 Pa for these formulations. High doses of radiation cause breakage in the guluronic acid blocks, thereby decreasing the ability of the alginate to form crosslinks [28]. Oxidation treatment renders alginate susceptible to hydrolysis and cleavage of alginate polymer chains, thereby decreasing the molar mass. Follicle growth, antrum formation, theca development, steroid production, relative hormone levels, and the ability of the oocytes to resume meiosis were all enhanced by culture within the oxidized and irradiated alginate relative to the unmodified alginate of the same solids concentration. We suggest that decreasing the stiffness of the alginate matrix allows for the maintenance of tensional homeostasis within the cellular compartments, thereby promoting the cellular processes that create a local paracrine milieu that increases oocyte quality.
The role of solids concentration was investigated by comparing 0.7% untreated alginate with the oxidized and irradiated alginate, all of which had similar stiffness. The oxidized, irradiated, and 0.7% alginate had similar shear moduli but differed in the solids concentration (1.5% vs. 0.7%). Decreasing the solids concentration of the matrices did not significantly affect growth or oocyte quality relative to oxidation or radiation treatment, but antrum formation was significantly enhanced in low concentration alginate for both stages of follicles. Decreasing the solids concentration may influence diffusion, either increasing availability of factors that promote cellular processes or allowing inhibitory factors to escape. In addition to antrum formation, decreasing the solids concentration led to an increase in estradiol production and decreased relative levels of androstenedione and progesterone. These relative hormone levels determined at 0.7% alginate represent the steroid profile of a healthy, growing follicle in vitro. Taken together, these experiments demonstrate that matrix stiffness substantially enhances follicle development and steroid production, which is further enhanced by the solids concentration of the matrix.
Our results demonstrate that the physical properties of alginate function to coordinate the differentiation of the multiple cellular compartments (oocyte, granulosa, and theca cells). Follicular cells communicate via secreted factors and gap junctions; thus, a mechanical stimulus or signal is quickly transmitted throughout the follicle population, a phenomenon observed in other engineered systems [31]. While mechanotransduction is a regulator of tissue development, the molecular basis of this process has only been investigated relatively recently, and much remains unknown [1, 32]. Mechanotransduction may be attributed to autocrine mechanoregulatory circuits in mechanoresponsive tissues [5, 33], and similar mechanisms may be in place in the ovary. Expression of other factors including fibroblast growth factor, PI 3-kinase, Akt, transforming growth factor beta (TGFβ) and matrix metalloproteinases (MMPs), among others, is also mediated by mechanical loading [3]. Additionally, tensional forces exerted by gel matrices may regulate cell aggregate morphogenesis via a GTPase-dependent mechanism [3, 5]. Any combination of mechanically-activated signaling pathways may contribute to the role of the matrix stiffness on follicle development and disease, suggesting a series of new lines of investigation. This study represents the seminal investigation into the role of mechanotransduction in ovarian follicle development.
The physical properties of the encapsulating alginate matrix represent a novel mechanism for the regulation of follicle development. To date, the factors known to regulate follicle development in vivo have been diffusible gonadotropins. However, the activity of these factors are likely context dependent, and the presentation of these factors to follicles entrapped within a matrix that has a non-permissive stiffness or density may be the fundamental mechanism that underlies some ovarian disorders. Specifically, the steroid profiles reported herein for stiff and dense matrices resemble trends observed in ovarian disease. The high level of androstenedione and progesterone in the immature follicles cultured under the ‘non-permissive’ condition (3%) suggests inadequate steroid conversion and is consistent with poor follicle health [34] and follicles from women with PCOS [35, 36]. Additionally, PCOS follicles have a significant amount of stroma and theca cell hypertrophy, and PCOS ovarian cortexes are thicker and more collagenized than normal ovaries [37]. A provocative interpretation is that the biomechanical environment of the PCOS ovary creates the in vivo equivalent of a ‘non-permissive’ mechanical environment and phenocopies the ‘stiff’ 3% alginate conditions (Figure 7).
Figure 7.
Proposed model for mechanical regulation of ovarian function and disease: (A) Immature follicles reside in the cortex in the human ovary. Unknown signals stimulate follicle development from the primordial to primary to secondary stage of development. We suggest that the biomechanics of the environment contribute to follicle development and that follicle selection may rely on a biomechanical signal from the surrounding cortex. The relatively dense cortex may maintain quiescence while the perimedullar interior of the ovary represents a more permissive biomechanical environment. (B) The follicles found in a PCOS ovary are usually small, accumulate in the cortex, and secrete high levels of androgen and relatively low levels of estrogen. The underlying etiology of this disease is unknown. Based on our work, we hypothesize that the relatively dense cortex creates a biomechanically non-permissive environment and is one of the contributing factors in the disease.
Conclusion
In this report, the stiffness and solids concentration of alginate hydrogels influenced the growth, differentiation, and paracrine communication between the cellular compartments of the follicle. A low-stiffness hydrogel is most permissive to follicle maturation, as demonstrated by follicle growth, cellular differentiation, appropriate steroid production, and oocyte quality. While effects of matrix stiffness on cells in three-dimensional culture have been well-documented, the concept of mechanical regulation of follicle development is novel and may have far reaching implications for understanding ovarian disease and preserving fertility. In addition, some cellular processes were independent of the stiffness, yet were dependent upon the solids content of the hydrogel, emphasizing that these and other physical properties must be considered in combination during the design of biomaterial matrices for tissue engineering.
Acknowledgments
The authors would like to thank Dr. Pamela Kreeger, Dr. Jeffrey Chang, and Dr. Walter Miller for helpful discussion, Monica Gomberg and Jason Deck for their assistance in follicle measurements, Dr. Tom Chiesl and the laboratory of Dr. Annelise Barron for assistance in GPC measurements, Dr. Wesley Burghardt for assistance in shear modulus measurements, and Dr. Hyun Joon Kong for assistance in alginate irradiation. Steroid assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by NIH U54 HD28934. This work is supported by NIH U54 HD41857.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28(2):134–46. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9(4):325–42. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 4.Marti A, Feng Z, Altermatt HJ, Jaggi R. Milk accumulation triggers apoptosis of mammary epithelial cells. Eur J Cell Biol. 1997;73(2):158–65. [PubMed] [Google Scholar]
- 5.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Oster GF, Murray JD, Harris AK. Mechanical aspects of mesenchymal morphogenesis. J Embryol Exp Morphol. 1983;78:83–125. [PubMed] [Google Scholar]
- 7.Trinkaus JP. Cells into organs: The forces that shape the embryo. 2. Englewood Cliffs (NJ): Prentice Hall College Div; 1984. [Google Scholar]
- 8.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32(3):407–17. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 9.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67(4):1430–6. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 10.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 11.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Engineering. 2003;9(5):1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 12.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27(5):714–23. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25(4):287–99. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11(12):2656–66. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 15.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Developmental Biology. 2005;279(1):20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73(2):351–357. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- 18.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12(10):2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 20.Gutowska A, Jeong B, Jasionowski M. Injectable gels for tissue engineering. Anat Rec. 2001;263(4):342–9. doi: 10.1002/ar.1115. [DOI] [PubMed] [Google Scholar]
- 21.Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating Bone Formation via Controlled Scaffold Degradation. J Dent Res. 2003;82(11):903–8. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 22.Bouhadir KH, Hausman DS, Mooney DJ. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer. 1999;40:3575–84. [Google Scholar]
- 23.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 24.Haug A, Larsen B, Smidsrod O. Studies of the sequence of uronic acid residues in alginic acid. Acta Chem Scand. 1967;21:691–704. [Google Scholar]
- 25.Haug A, Larsen B. Quantitative determination of the uronic acid composition of alginates. Acta Chem Scand. 1962;16:1908–18. [Google Scholar]
- 26.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73(5):942–50. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 28.Kong HJ, Smith MK, Mooney DJ. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24(22):4023–9. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 29.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17(5):945–50. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 30.Kong HJ, Lee KY, Mooney DJ. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer. 2002;43:6239–46. [Google Scholar]
- 31.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci U S A. 2001;98(11):6180–5. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20(7):811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 33.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429(6987):83–6. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson GF, Magoffin DA, Garzo VG, Cheung AP, Chang RJ. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod. 1992;7(3):293–9. doi: 10.1093/oxfordjournals.humrep.a137638. [DOI] [PubMed] [Google Scholar]
- 35.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83(11):3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 36.Joseph-Horne R, Mason H, Batty S, White D, Hillier S, Urquhart M, et al. Luteal phase progesterone excretion in ovulatory women with polycystic ovaries. Hum Reprod. 2002;17(6):1459–1463. doi: 10.1093/humrep/17.6.1459. [DOI] [PubMed] [Google Scholar]
- 37.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37(2):59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]



