Abstract
Previous findings from our laboratory demonstrated that some clonally expanded cerebrospinal fluid (CSF) B cells from MS patients exhibit diminished mutation targeting patterns in comparison to typical B cells selected in the context of germinal centers (GCs). In order to determine whether the overall CSF B cell repertoires adhered to mutation patterns typical of GC-selected B cells, we analyzed the immunoglobulin repertoires from CSF B cells of 8 MS patients for mutation characteristics typical of GC-derived B cells. Mutation targeting was preserved. Thus, clonal expansion of some CSF B cells may occur independently of GC, but the CSF B cell pool is governed by typical GC selection. Interestingly, the heavy chain CDR3’s of CSF B cells from MS patients had a net acidic charge, similar to GC-derived B cells, but a tendency towards longer CDR3’s, consistent with autoreactive B cells. How these findings may support current hypotheses regarding the origin of CSF B cells is discussed.
Keywords: Multiple Sclerosis, cerebrospinal fluid, B lymphocytes, immunoglobulin rearrangements, mutation accumulation
INTRODUCTION
Clonal expansion of CSF B cells in MS patients, including those patients recently diagnosed with MS, is readily observed, and suggests that at least some of the B cells in the CSF of MS patients are being driven by antigen(s) present in the CNS compartment (Blalock et al., 1999; Columbo et al., 2000; Monson et al., 2005; Owens et al., 2003; Qin et al., 1998; Ritchie et al., 2004). Our laboratory has documented that clonally expanded CSF B cells from MS patients have unusually high mutational frequencies and in most cases, lack enhanced targeting of mutations to the RGYW/WRCY motifs in heavy and light chain CDRs in comparison to Healthy Control Peripheral Blood (HCPB) B cells (Monson et al., 2005). These data were surprising since targeting of mutations to CDRs, and more specifically, to RGYW/WRCY motifs within CDRs are characteristic features of germinal center reactions (Jolly et al., 1996; Monson et al., 2001; Neuberger et al., 1998; Neuberger and Milstein, 1995; Rada et al., 1998) driven by antigen encounter. Thus, we hypothesized that clonally expanded CSF B cells are not necessarily governed by classical germinal center reactions.
However, several studies suggested that the CNS itself can provide a “germinal center like” environment (Harling-Berg et al., 1989; Hochwald et al., 1988; Knopf et al., 1995; Knopf et al., 1998; Phillips et al., 1997; Prinease, 1979; Sellebjerg et al., 2000; Torcia et al., 2001; Widner et al., 1988). More recent findings have demonstrated that 1) the CNS harbors germinal center structures in the meninges of MS patient brain samples that contain severely demyelinated lesions (Serafini et al., 2004), 2) B cells (centroblasts and centrocytes) which reside specifically in germinal centers are present in the CSF of MS patients (Corcione et al., 2004), and 3) high levels of chemokines and cytokines that support germinal center formation and function (CXCR3, LT-α, CXCL12, and CXCL13) are also present in the CSF of MS patients (Corcione et al., 2004; Sorensen et al., 2002). Furthermore, evidence of intraclonal diversity among CSF B cell clones of MS patients (Monson et al., 2005) also substantiates that the CSF supports a GC-like environment, since intraclonal diversity is most often observed in GC follicles. Nevertheless, our observations regarding mutation patterns in clonally expanded CSF B cells from MS patients would suggest that even though the CNS can support GC follicle formation and contain a GC supportive environment, GCs may not be required for differentiation and maturation of clonally expanded CSF B cells.
The current study was undertaken to determine whether the inclusive CSF B cell antibody repertoires from MS patients also deviate from typical mutation patterns of GC-derived B cells, as do some clonally expanded CSF B cells from MS patients. If the inclusive CSF B cell repertoires from MS patients also deviated from mutation patterns characteristic of GC-derived B cells, then the hypothesis that GCs may not be required for differentiation and maturation of clonally expanded CSF B cells could be extended to the CSF B cell populations as a whole as well. In order to address this issue, we analyzed the CSF B cell antibody repertoires from eight MS patients for mutational characteristics typical of GC-selected B cells. We found that the CSF B cell antibody repertoires from eight MS patients adhere to mutation patterns typical of classic GC reactions, indicating that the majority of CSF B cells had likely been selected in the context of a GC. These observations contrast with what we had observed in clonally expanded CSF B cells from MS patients, which did not uniformly demonstrate mutational targeting characteristics typical of GC selected B cells, especially as they relate to RGYW/WRCY targeting to CDRs. Hence, it is likely that although some examples of clonal expansion may occur outside the context of a GC, CSF B cell populations from MS patients as a whole are governed by GC selection.
Finally, the immunoglobulin repertoires of self-reactive B cells are enriched for longer heavy chain CDR3s that have accrued basic (positively charged) amino acid residues (Wardemann et al., 2003), but are subsequently removed as B cell development progresses in a non-autoimmune setting. However, in the autoimmune setting of Systemic Lupus Erythmatosis (SLE), long heavy chain CDR3s with accruals of positively charged amino acids are selected into the ANA polyreactive B cell repertoire (Yurasov et al., 2005). These data indicated that there is a breach in normal B cell selection in SLE B cell repertoires at the time that these cells should be undergoing selection in the context of a germinal center. Interestingly, heavy chain CDR3s from CSF B cells of these MS patients were also longer than normal GC-selected B cells, but nevertheless, maintained net acidic charges indicative of GC-selected B cells.
MATERIALS and METHODS
Patient Description
Fourteen patients with Clinically Definite Multiple Sclerosis (CDMS)(Table 1) and seven patients who did not have CDMS (Table 2) were recruited for this study in accordance with UT Southwestern Institutional Review Board. The non-CDMS population used in this study is somewhat heterogenous although for clarity purposes here, are categorized as other neurological disease (OND), with OND subclasses. Three would be considered non-inflammatory neurological diseases unrelated to MS (OND 142, 758 and 766), two would be considered other inflammatory neurological disease (OND 341 and 658), and two would be considered CIS (OND 132 and 681). CIS 132 converted to clinically definite MS 18 months after this sampling.. Eight of the 10 MS patients used in the flow cytometry analysis and 3 of the 7 non-CDMS patients (OND 341 and 758, CIS 132) had antibody databases that could be further analyzed for mutation characteristics. The HCPB antibody database has been used extensively for comparison purposes in other studies (Dorner et al., 1998a; Foster et al., 1999; Monson et al., 2000) and consists of 232 heavy chain rearrangements and 133 kappa chain rearrangements from peripheral blood B cells of two healthy donors.
Table 1.
MS Patient Summary1
| M1252,3,4,5 | M2172 | M3544,5 | M3683,4,5 | M3762,3,4 | M4844,5 | M5222,3,4 | M5582 | M5843,4 | M8752,3,4,5 | M8872 | M9182 | M9272 | M9442 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of MS | RR | RR | RR | RR | RR | PP | RR | RR | RR | RR | RR | RR | RR | SP |
| Time since MS diagnosis | <1 year | 18 years | <1 year | 15 years | 20 years | 3 months | 3 years | 1 year | 1 month | 13 years | 10 years | 8 years | 10 years | |
| Age/Sex | 32/F | 45/F | 44/F | 41/F | 56/F | 46/F | 35/F | 29/F | 44/F | 35/F | 50/F | 38/M | 31/F | 53/F |
| Exacerbation History | ON | Dystonia | TM | TM | ON | Myelitis | TM | ON | TM | ON | ON | Myelitis | ON | ON |
| MRI Findings | GD+ | WML | WML | WML | WML | WML | GD+ WML | GD+ WML | GD+ | GD+,WML | WML | WML | WML | WML |
| Clonal Expansion | Yes | n.d. | Yes | Yes | Yes | Yes | Yes | n.d. | Yes | Yes | No | n.d. | n.d. | n.d. |
| Oligoclonal Bands | No | n.d. | Yes | Yes | No | Yes | Yes | n.d. | n.d. | n.d. | No | No | No | Yes |
| Ig Index | NL | High | NL | High | n.d. | High | High | n.d. | n.d. | n.d. | NL | NL | NL | High |
| Ig Synthesis | n.d. | n.d. | NL | High | n.d. | High | n.d. | n.d. | n.d. | n.d. | NL | NL | NL | High |
RR; Relapsing Remitting, SP; Secondary Progressive, ON; Optic Neuritis, TM; Transverse Myelitis, GD+; gadolinium enhancing, WML; White Matter Lesions
All patients had CSF white blood cell (WBC) counts in the range of 1 X103 to 1 X104 per mL, typical of MS patients at UTSWMC (Stuve, Ann. Neurol., 2006)
Patient data used in flow cytometric analysis
Patient data used in repertoire analysis
Patient data used in mutation analysis and heavy chain CDR3 charge analysis
Patient clonal analysis previously published in Monson et al, JNI, 2005
Table 2.
OND Patient Summary1
| CIS 132 | OND 142 | OND 341 | OND 658 | CIS 681 | OND 758 | OND 766 | |
|---|---|---|---|---|---|---|---|
| OND subcategory | CIS | NIND | OIND | OIND | CIS3 | NIND | NIND |
| Age/Sex | 24/F | 32/F | 70/M | 30/F | 53/F | 45/F | 33/F |
| Presentation or Diagnosis at time of sampling | Diplopia2 | HA | Ataxia, PS | Ataxia | Neuroma | HA | HA |
| MRI Findings | GD+ | WML | No Lesions | No lesions | WML | WML | WML |
| Clonal Expansion | No | No | No | n.d. | No | Yes | No |
Abbreviations: OND; Other Neurological Disease, CIS; Clinically Isolated Syndrome, NIND; non-inflammatory neurological disease, OIND; Other Inflammatory Neurological Disease, HA; Headache, PS; Paraneoplastic Syndrome, GD+; Gadolinium Enhancing, WML; White Matter Lesions
All patients had CSF white blood cell (WBC) counts typical of OND controls at UTSWMC (Stuve, Ann. Neurol., 2006)
This patient converted to clinically definite MS according to the Poser Criteria 18 months after sampling.
This patient’s CSF analysis was negative for oligoclonal bands, and normal for Ig synthesis and rate.
Cell preparation and cell sorting
These methods were carried out as previously described (Monson et al., 2005). B cells were sorted based on their CD19 expression only using either the MoFlo High-Performance Cell Sorter (Cytomation, Ft. Collins, CO), or the FACS Diva flow cytometer (Becton Dickinson, San Jose, CA) outfitted with an automated cell deposition unit, which deposited one cell per well in 96 well plates. CD19 and CD27 antibodies were purchased from Becton Dickinson (San Jose, CA).
Primer extension preamplification, amplification of VHDJH, V•J• and VλJλ rearrangements and sequence analysis
These methods were carried out as previously described (Brezinschek et al., 1995; Farner et al., 1999b; Foster et al., 1997). Heavy chain D segments were identified using the Junction Analysis tool (Yousfi et al., 2004). Comparison of sequence variation and mutational characteristics between naïve and memory CSF B cells was not addressed here since cells were sorted based on CD19 expression alone which allowed for direct comparisons to HCPB B cell repertoires. VH and Vk family frequencies were only calculated for those CSF B cell repertoires that consisted of more than 15 rearrangements.
Non-clonal and Clonal Population Definitions
Clonally expanded CSF B cells are defined as those single B cells whose heavy and light chain rearrangements were represented two or more times in the repertoire. The non-clonal CSF B cell population is defined as those single B cells whose heavy and light chain rearrangements were not represented more than once in the repertoire. Inclusive CSF B cell populations (which were analyzed here) consist of both non-clonal and clonal CSF B cell immunoglobulin rearrangements.
Determination of VH CDR3 Charges
Heavy chain CDR3 regions include the entire DH segment and JH segment through codon 102. CDR3 regions may include up to 2 nucleotides from the VH segment to allow for in frame analysis. The net charges of heavy chain CDR3 regions were analyzed using Lasergene software (DNASTAR Inc., Madison, WI) to translate nucleotide sequence into amino acid sequence and to determine total average charge of the heavy chain CDR3 translated region at pH 7.
Statistical Methods
Fisher’s Exact Test was used to compare the distribution of heavy and light chain rearrangements found in the productive repertoires of the MS patients. P-values equal to or less than 0.05 were considered significant. Mutational frequencies (MF) were compared using the χ2 test. VH CDR3 lengths were compared using the mann-whitney t-test.
RESULTS
Memory B cells are enriched in the CSF B cell pool of MS and OND patients
We had previously reported that extensive clonal expansion and high mutational frequencies in the clonally expanded CSF B cell population from MS patients likely indicated that most CSF B cells are of a memory phenotype, rather than naïve (Monson et al., 2005). Indeed, others have now confirmed by flow cytometric methods that the majority of CSF B cells are of a memory phenotype (CD27+)(Cepok et al., 2005; Corcione et al., 2004). The CSF B cell profiles (memory vs naïve) from patients analyzed for this study also adhered to this observation. For example, memory B cells in the CSF of MS patients were enriched compared to the peripheral blood from the same MS patient (compare 20.6 ± 4.4% memory B cells in PB to 55.3 ± 7.8% memory B cells in CSF, Figure 1). The CSF B cell profiles (memory vs naïve) in the OND patients were also enriched for memory B cells compared to PB (compare 17.0 ± 2.9% memory B cells in PB to 53.8 ± 13.4% memory B cells in CSF, Figure 1). In fact, memory B cells were so prevalent in the CSF of both the MS and OND patients, that the typical ratios of naive:memory B cells in CSF of both MS and OND patients was inverted in comparison to PB (Table 3). Interestingly, the two CIS patients also had a higher propensity towards memory B cell enrichment in their CSF compared to the PB (Figure 1).
Figure 1. Frequency of B cell subsets in PB and CSF of OND and MS patients.

Cells isolated from PB and CSF of MS patients (n=10) and OND patients (n=7) were analyzed by flow cytometry using anti-CD19 and anti-CD27 mAbs. Total CD19+ cells (defined as a percentage of total lymphocytes) were analyzed for expression of CD27 to determine naïve (CD27−) and memory (CD27+) B cell subsets. Average frequencies (±standard error) of each subset are as indicated below the x-axis.
Table 3.
Naïve:Memory Ratios of B cells from Flow Cytometric Analysis
| MS | OND | Comparison of MS to OND | |
|---|---|---|---|
| PB Naïve B:Memory B | 3.8 | 4.8 | p=0.51 |
| CSF Naïve B:Memory B | 0.8 | 0.9 | p=0.84 |
|
| |||
| Comparison of PB to CSF | p= 0.0001 | p= 0.0001 | |
Heavy chain and Kappa Chain Family Usage is intact in the CSF B cell pool of MS patients
Since the CSF B cells consisted largely of memory B cells (which have already undergone antigen driven selection), we predicted that their antibody repertoires might be skewed from HCPB, which is largely naïve and not driven by antigen selection. In order to test this hypothesis, we analyzed the frequency of heavy and light chain rearrangement usage from the CD19+ CSF B cell populations of MS (n=8), one CIS and two OND patients.
In HCPB, VH3 and VH4 family members are more often utilized in heavy chain rearrangements, followed by VH1, and the other families (VH2, 5 and 6), as shown in Figure 2A. The CSF B cell repertoires from M522, M584, and M875 followed this pattern (VH3+4>1>2=5=6). However, M368 utilized VH1 V genes more often than any other family (VH1>3>4>2=5=6), which is likely attributed to the contribution of several clones utilizing the VH1 family gene, 1–69 in this repertoire. In HCPB, JH4 family members are more often utilized in heavy chain rearrangements, followed by JH6, and the other families (JH5, 3, 2 and 1), as shown in Figure 2A. The frequency of JH4 usage in the CSF B cells of the OND patients was similar to that of HCPB (p=0.98), but there was a significant decrease in JH4 segment usage in the CSF B cell heavy chains from MS patients (Figure 2B) in comparison to HCPB (36% vs 54%, p=0.0001). Furthermore, M368, M522, and M584 had an increased frequency of JH5 in comparison to HCPB. Increases in distal J segment usage are characteristic of receptor editing (Nemazee, 2000), thus indicating that the heavy chain repertoire of MS (but not OND) patients may have undergone receptor editing to some degree
Figure 2. Heavy and Kappa Variable and Junction Chain usage in HCPB, OND CSF, and MS CSF B cells.

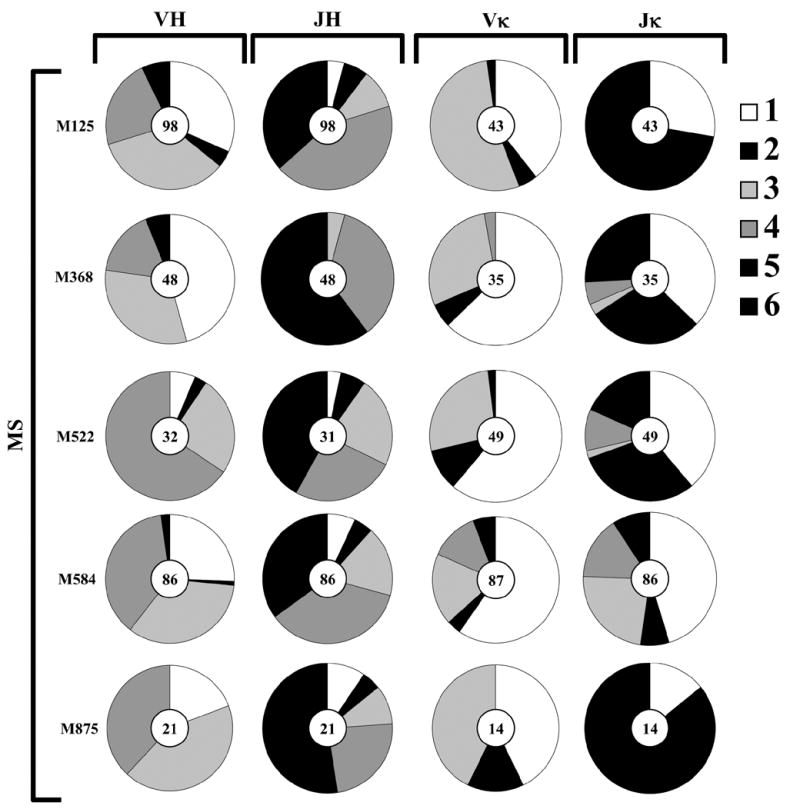
Antibody rearrangements were analyzed from single CSF B cells as described in Materials and Methods from MS one CIS and two OND patients as indicated. (A) Heavy(H) and Kappa(κ), Variable(V) and Junction(J) segment usage in HCPB and CSF CD19+ B cells are presented as a percentage of total sequences obtained from each patient sample. Center oval “n” indicates number of productive sequences obtained and analyzed. H chain V- and J-segment data is summarized on the left, κ chain V- and J-segment data on the right. (B) H and κ chain, V and J segment usage in CSF B cells from MS patients. Data is presented as indicated in Panel A.
Kappa light chain and J kappa segment usage in the CSF B cell populations are also similar in MS and OND patients compared to HCPB (Figure 2)(Foster et al., 1997).
Enhanced Mutational Frequency of CSF B cell repertoires of MS patients
Mutated VH rearrangements are characteristic of memory B cells (Klein et al., 1998). Since the majority of CSF B cells in MS, CIS and OND patients were of a memory (CD19+CD27+) phenotype, we predicted that the CSF B cell antibody repertoires would have a substantial accumulation of mutations such that the overall mutational frequencies (MF) would be greater than what was observed in HCPB (Dorner et al., 1997; Dorner et al., 1998b; Dorner et al., 1998c), since HCPB B cells are largely naive. Indeed, six of the eight heavy chain antibody repertoires from CSF B cells of MS patients had significantly higher mutational frequencies (MF) than HCPB (Table 4). In fact, the average MF of all MS patients in this analysis was 5.0, which is 1.5 fold greater than HCPB. This observation is further emphasized by a separate analysis of just those CSF Ig heavy chains that are mutated which generates a MF of 5.8, which is 1.8 fold greater than HCPB. This was also the case with CIS 132 and OND 341, which likely reflects the observation that CSF from patients have a high prevalence of memory B cells, rather than naïve. Indeed, over 86% of the heavy chains in this analyses have accumulated considerable mutations, and are thus likely not naïve B cells.
Table 4.
Overall Mutational Frequencies from Heavy and Kappa Chain CSF B cells
| Heavy Chain | Kappa Chain | |||||
|---|---|---|---|---|---|---|
| n | MF (×10−2) | Comparison to HCPB | n | MF (×10−2) | Comparison to HCPB | |
| HCPB | 3.34 | NA | 2.6 | NA | ||
|
| ||||||
| CIS132 | 17 | 4.8 | ^ | 15 | 3.8 | ^ |
| OND341 | 32 | 6.9 | ^ | 20 | 2.9 | = |
| OND758 | 18 | 3.0 | = | 16 | 1.8 | ∨ |
|
| ||||||
| M125 | 100 | 4.9 | ^ | 43 | 2.2 | ∨ |
| M354 | 6 | 6.1 | ^ | 1 | n.d. | n.d. |
| M368 | 48 | 6.6 | ^ | 35 | 3.4 | ^ |
| M376 | 8 | 7.2 | ^ | 6 | n.d. | n.d. |
| M484 | 9 | 9.5 | ^ | 8 | n.d. | n.d. |
| M522 | 32 | 6.4 | ^ | 49 | 2.4 | = |
| M584 | 86 | 3.7 | = | 87 | 3.3 | ^ |
| M875 | 21 | 1.4 | ∨ | 14 | n.d. | n.d. |
| AVE MS | 5.0 | ^ | 2.9 | ^ | ||
The kappa chain MF comparison was more variable than the heavy chain MF comparisons (Table 4). Some of the kappa chain repertoires from the CSF B cells of MS patients demonstrated a higher MF than in HCPB (M368 and M584), but one was similar to HCPB (M522), and still another was lower (M125). The OND population demonstrated a similar lack of consistency, one kappa chain repertoire (OND 341) had a similar MF, and OND 758 had a lower MF than HCPB. Interestingly, CIS 132, had a higher MF than HCPB,. The average MF of the MS CSF kappa chain repertoires combined had a slightly higher MF compared to HCPB (2.9 vs 2.6, p=0.019).
Targeting of mutations to CDRs is intact in the CSF B cell repertoires of MS patients
Mutation targeting to CDRs is characteristic of B cells that have been selected and activated in the context of a germinal center (Dorner et al., 1997; Dorner et al., 1998a; Dorner et al., 1998b; Dorner et al., 1999; Dorner et al., 1998c; Farner et al., 1999a; Foster et al., 1997; Foster et al., 1999). However, some of the clonally expanded CSF B cells from MS patients characterized in our laboratory had diminished targeting to CDRs, despite high mutational frequencies overall. We hypothesized that this diminished targeting to CDRs would be unique to clonally expanded CSF B cells from MS patients, such that the CSF B cell repertoire as a whole, would adhere to normal mutation accumulation patterns. In order to test this hypothesis, we calculated the percentage of mutations that were present in CDRs of the inclusive CSF B cell heavy and kappa chain repertoires from MS patients, comparing them to values we had established in HCPB B cells (Table 5).
Table 5.
Percent mutations in CDRs of Heavy and Kappa chains from CSF B cells
| Heavy Chain | Kappa Chain | |||||
|---|---|---|---|---|---|---|
| n | %M in CDR | Comparison to HCPB | n | %M in CDR | Comparison to HCPB | |
| HCPB | 484 | 47.0 | NA | 754 | 55.0 | NA |
|
| ||||||
| CIS132 | 182 | 52.2 | = | 124 | 58.1 | = |
| OND341 | 452 | 45.8 | = | 129 | 58.9 | = |
| OND758 | 123 | 45.5 | = | 64 | 59.4 | = |
|
| ||||||
| M125 | 891 | 53.3 | ^ | 224 | 60.7 | = |
| M354 | 73 | 46.6 | = | 2 | n.d. | n.d. |
| M368 | 643 | 56.0 | ^ | 263 | 52.9 | = |
| M376 | 95 | 51.6 | = | 37 | n.d. | n.d. |
| M484 | 164 | 545.7 | = | 75 | n.d. | n.d. |
| M522 | 439 | 49.2 | = | 250 | 64.8 | ^ |
| M584 | 650 | 64.5 | ^ | 632 | 61.1 | ^ |
| M875 | 64 | 37.5 | = | 7 | n.d. | n.d. |
| AVE MS | 3019 | 54.7 | ^ | 1369 | 60.1 | ^ |
Frequencies of mutation in heavy or kappa chain CDRs from CSF B cells of all 8 of the MS patients were either similar or greater than values established in HCPB (Table 5). For example, 47% of mutations accumulated in the CDRs of heavy chain rearrangements from HCPB. All eight MS CSF B cell heavy chain antibody repertoires demonstrated targeting of mutations to CDRs at levels similar to HCPB or higher (54.7% average for MS CSF VH rearrangements vs 47% HCPB VH rearrangements, p=0.0001). Hence, CSF B cells from MS patients appear to have been selected in the context of a GC. The two OND and one CIS heavy chain antibody repertoires also demonstrated targeting of mutations to CDRs at levels similar to HCPB, indicating that CSF B cells from non-MS patients had also been selected in the context of a germinal center.
All of the kappa chain CSF Ig repertoires whether derived from MS patients or not demonstrated targeting of mutations into CDRs at similar or higher levels as HCPB (Table 5). The demonstration of intact targeting of mutations to the CDRs further confirms that these B cells were subject to normal germinal center selection, even in MS patients whose repertoires may be more biased towards autoreactivity.
Bias towards Replacement Mutations in CDRs of heavy and kappa chain repertoires is intact in the CSF B cell repertoires of MS patients
Mutations resulting in a change in the amino acid sequence (termed “replacement” mutations), are more likely to accumulate in the CDRs rather than FRs (Dorner et al., 1997; Dorner et al., 1998a; Dorner et al., 1999; Farner et al., 1999a; Foster et al., 1999), presumably because diversity in CDRs results in greater variability in antigen binding and affinity, whereas diversity in FRs would result in compromised structural integrity of the antibody (Shlomchik et al., 1987). We reasoned that since targeting of mutations to CDRs was intact in the CSF B cell repertoires, then we would likely observe an accumulation of replacement mutations in the CDRs compared to the FRs as well.
Replacement:Silent (R:S)(“silent” mutations do not result in amino acid change) ratios of mutations within the CDRs and FRs were calculated for each of the eight MS, one CIS and two OND heavy chain repertoires (Table 6). As indicated, the heavy chain repertoires from the CSF B cells of all eight MS patients had significantly higher R:S ratios in the CDRs compared to the FRs. The heavy chain repertoires of CIS 132 and OND 341 also demonstrated higher R:S ratios in the CDRs compared to the FRs. A bias in replacement mutations accumulating into CDRs of kappa rearrangements was also observed (Table 6) in all four MS patients, as well as CIS 132 and OND 758. Therefore, the mechanism that targets replacement mutations into the CDRs rather than in FRs of heavy and light chains is largely intact in CSF B cell repertoires from MS patients, and some non-MS patients.
Table 6.
Replacement:Silent Ratio’s in Heavy and Kappa Chains
| Heavy Chain R:S | Kappa Chain R:S | |||||
|---|---|---|---|---|---|---|
| CDR | FR | Targeting to: | CDR | FR | Targeting to: | |
| OND132 | 3.4 | 1.4 | CDR | 3.1 | 1.3 | CDR |
| OND341 | 5.4 | 2.0 | CDR | 3.1 | 2.5 | = |
| OND758 | 3.9 | 2.0 | = | 4.1 | 0.9 | CDR |
|
| ||||||
| M125 | 3.6 | 1.5 | CDR | 3.6 | 1.7 | CDR |
| M354 | 7.3 | 1.8 | CDR | n.d. | n.d. | n.d. |
| M368 | 4.3 | 1.6 | CDR | 4.9 | 1.6 | CDR |
| M376 | 4.8 | 1.5 | CDR | n.d. | n.d. | n.d. |
| M484 | 4.6 | 1.9 | CDR | n.d. | n.d. | n.d. |
| M522 | 4.2 | 1.2 | CDR | 3.4 | 1.4 | CDR |
| M584 | 5.4 | 1.1 | CDR | 3.7 | 1.4 | CDR |
| M875 | 2.2 | 0.7 | CDR | n.d. | n.d. | n.d. |
| AVE MS | 3.6 | 1.4 | CDR | 3.8 | 1.2 | CDR |
Targeting of Mutations to RGYW/WRCY motifs in CDRs is intact in the CSF B cell repertoires of MS patients
Mutations in the heavy and light chain repertoires of HCPB B cells are also targeted to particular motifs that follow the amino acid sequence, RGYW or WRCY, and the majority of mutations within CDRs are contained within these motifs (Dorner et al., 1999; Dorner et al., 1998c; Farner et al., 1999a; Foster et al., 1999). We predicted that since mutations in the CSF B cell repertoires were targeted to CDRs, the next “level” of targeting—that is, to RGYW/WRCY motifs—would also be conserved in the heavy and kappa rearrangements of the CSF B cell repertoires.
Mutations within the RGYW/WRCY motifs in CDRs of HCPB heavy chain rearrangements represent 60% of all mutations in CDRs. In contrast, only 19% of mutations in FRs of HCPB heavy chain rearrangements are within RGYW/WRCY motifs. This indicates that mutations within RGYW/WRCY motifs are targeted to the CDRs (Farner et al., 1999a). Targeting of mutations to RGYW/WRCY motifs was intact in 5 of the 8 CSF B cell repertoires from MS patients (as well as CIS 132 and both OND patients)(Table 7), In addition, the average percentage of mutations in RGYW/WRCY of CDRs of all 8 MS patients combined was no different than HCPB (compare 57% MS average to 60% HCPB average, p=0.465). However, when only the heavy chain rearrangements from clonally expanded CSF B cells were considered (Table 8), the majority of clones demonstrated targeting of mutations to CDRs, but very few of the clones demonstrated targeting of mutations within RGYW/WRCY motifs in the CDRs.
Table 7.
Percent Mutations in RGYW/WRCY motifs of Heavy and Kappa Repertoires from CSF B cells
| Heavy Chain | Kappa Chain | |||||||
|---|---|---|---|---|---|---|---|---|
| CDR | FR | CDR | FR | |||||
| %M in R/W | Comparison to HCPB | %M in R/W | Comparison to HCPB | %M in R/W | Comparison to HCPB | %M in R/W | Comparison to HCPB | |
| HCPB | 60 | NA | 19 | NA | 60 | NA | 49 | NA |
|
| ||||||||
| OND132 | 58.3 | = | 28.2 | = | 59.7 | = | 38.8 | = |
| OND341 | 53.9 | = | 39.2 | ^ | 60.7 | = | 49.0 | = |
| OND758 | 47.7 | = | 33.3 | = | 36.1 | = | 47.8 | = |
|
| ||||||||
| M125 | 64.3 | = | 41.9 | ^ | 65.2 | = | 53.4 | = |
| M354 | 42.4 | ∨ | 14.7 | = | n.d. | n.d. | n.d. | n.d. |
| M368 | 63.3 | = | 35.2 | ^ | 54.0 | = | 51.6 | = |
| M376 | 51.4 | = | 47.4 | ^ | n.d. | n.d. | n.d. | n.d. |
| M484 | 58.2 | = | 40.0 | ^ | n.d. | n.d. | n.d. | n.d. |
| M522 | 62.4 | = | 45.3 | ^ | 53.8 | = | 30.7 | ∨ |
| M584 | 45.4 | ∨ | 32.3 | ^ | 55.4 | = | 44.7 | = |
| M875 | 36.8 | ∨ | 31.4 | = | n.d. | n.d. | n.d. | n.d. |
| AVE MS | 57.7 | = | 37.9 | ^ | 56.5 | = | 45.4 | = |
Table 8.
Mutation targeting summary of heavy chain rearrangements from CSF B cell clones
| Targeting to CDR | Targeting to RGYW/WRCY motifs within CDRs | |||
|---|---|---|---|---|
| N | Percent | n | Percent | |
| M125 | 6/7 | 85 | 2/7 | 29 |
| M368 | 5/7 | 71 | 2/7 | 29 |
| M522 | 4/8 | 50 | 3/8 | 38 |
| M584 | 10/15 | 67 | 8/15 | 53 |
Of note, 6 of the 8 MS CSF B cell heavy chain repertoires exhibited an increase in the frequency of RGYW/WRCY mutations in the FRs that was not observed in HCPB (Table 7). For example, 42% of FR mutations of M125 were within RGYW/WRCY motifs. This is significantly greater than HCPB, in which only 19% of FR mutations are within RGYW/WRCY motifs (p=0.0001). This increased frequency of FR mutations within RGYW/WRCY motifs was also evident in the MS average (compare 38% MS average to 19% HCPB average, p=0.0001), and cannot be attributed to lack of targeting to CDRs, since this aspect of VH rearrangement is similar in HCPB and MS (and OND or CIS) CSF B cells. Targeting of RGYW/WRCY mutations in the CDRs and FRs of kappa chain repertoires from MS, CIS OND CSF B cells was no different than HCPB (Table 7).
CSF B cells from MS patients have longer VH CDR3s than HCPB
It is suspected that there is a breach in B cell selection in SLE B cell repertoires derived from germinal centers such that B cells reactive to several self antigens (i.e. ANA polyreactive B cells) are actually selected into the B cell repertoire rather than removed (Yurasov et al., 2005). In fact, ANA polyreative B cells from SLE patients have longer VH CDR3 lengths than non-reactive mature GC selected B cells (Wardemann et al., 2003). It became of interest then to determine if the VH CDR3 length of CSF B cells from MS patients was also increased. Indeed, the average VH CDR3 length in CSF B cells from these MS patients was 13.9 ± 0.29 amino acids (AA), which was significantly longer than the average VH CDR3 length of PB B cells from HCPB (p=.004)(Figure 3), but similar to that of ANA polyreactive B cells from SLE patients. Thus, longer VH CDR3 lengths are not unique to ANA polyreactive B cells, Interestingly, the average heavy chain CDR3 length of CIS 132 was significantly shorter than that of the 8 MS and 2 OND CSF B cell populations. Whether these longer VH CDR3 lengths of CSF B cells from MS patients can be associated with autoreactivity (as it has been with ANA polyreactive B cells) remains to be determined.
Figure 3. Heavy Chain CDR3 amino acid length.

CDR3 lengths from the heavy chains of CSF B cells from 8 MS, one CIS and two OND patients and HCPB were determined and separated into 4 groups of varying length. Average CDR3 lengths are listed above (+/− standard error of the mean, * indicates significance of p•.05, mann-whitney t-test).
VH CDR3 net charge composition is intact in the CSF B cell repertoires of MS patients
ANA polyreactive B cells also have a high propensity towards accrual of basic charges in their heavy chain CDR3s, presumably to enhance binding of DNA, which is acidic (Wardemann et al., 2003). Hence, one approach to determining if a patient may be prone to this autoimmune state would be to analyze the heavy chain CDR3 charge compositions.
We calculated the net charges of the CDR3 regions from the heavy chain rearrangements of the eight MS patients (Figure 4) and compared the results to the mature B cell repertoire defined by Wardemann et al (Wardemann et al., 2003) (henceforth referred to as “mature B cell population”). The majority (70% average) of heavy chain CDR3s from the eight MS CSF B cell antibody repertoires had net acidic charges in the range of −1 to −5. Approximately 80% of the mature B cell population had net acidic charges in the heavy chain CDR3, which was similar to the MS repertoires (p=0.27), with a charge range of −1 to −4. Those heavy chain CDR3s that were not net acidic were a mixture of no net charge “0” and some basic net “+” charges. Thus, the net charge composition of heavy chain CDR3s from CSF B cells of MS patients is similar to the mature B cell population.
Figure 4. Heavy Chain CDR3 total amino acid charge.

Amino acid sequence of heavy chain CDR3s of the CSF B cells isolated from MS, CIS and OND patients were analyzed to determine total CDR3 charge at pH 7. Charges are grouped from each patient repertoire and listed as a proportion of total sequences that were analyzed from each patient. Center circle “n” indicates number of sequences included in the analysis.
Given these observations, it was of interest to investigate whether the two OND and one CIS patient CSF B cell repertoires aligned with the MS patients, mature B cells and/or ANA polyreactive B cells with regard to net acidic charge of heavy chain CDR3s. Interestingly, the frequency of net acidic charged heavy chain CDR3s from the two OND and CIS patient CSF B cell antibody repertoires was decreased in comparison to the MS patients (p=XXX)(Figure 4). In fact, approximately half of the heavy chain CDR3s had net acidic charges “−”, and the other half had no net charge “0”. This indicated that heavy chain rearrangements with net acidic charges in their CDR3s are more prevalent in the CSF B cell heavy chain repertoires of patients with clinically definite MS and mature peripheral B cells from healthy donors, compared to the CSF B cell heavy chain repertoires of non-MS patients. This selection could not be attributed to bias in D segment usage (which constitutes a portion of the CDR3), as the frequency of D segment distributions was no different in the MS patients compared to the OND patients (data not shown). Of note, the only MS heavy chain CDR3 analysis that was similar to the OND/CIS pattern (half net acidic, half net no charge) was M368, an observation that we cannot explain at this time either clinically or by Ig repertoire analysis.
The B cell antibody repertoires from patients with Optic Neuritis (ON)(also categorized as Clinically Isolated Syndrome, and which commonly convert to CDMS) have been reported and demonstrate clonal expansion (Haubold et al., 2004). For comparison purposes, we calculated the net charges of the CDR3s from the heavy chain rearrangements of the CD19+ CSF B cell population reported for one of these patients (ON03-5, Table 3 of (Haubold et al., 2004)) and compared it to the frequencies of net acidic heavy chain CDR3s from our MS, CIS and OND patients. Interestingly, only 50%(n=32) of heavy chain CDR3s in the CSF B cell population from this ON patient were acidic, indicating a lack of skewing towards net acidic heavy chain CDR3s in this patient, just as we had observed in CIS132 and two OND patients. Furthermore, this frequency was statistically lower than that of MS patients (50% vs 70%, p=0.01), again emphasizing that acidic heavy chain CDR3s are not enriched in CSF B cells from these non-MS patients. Of note, this particular ON patient had only one lesion by MRI. It would be interesting to determine whether the ON patient in the Haubold study that had 17 lesions by MRI (ON03-3) would have a frequency of acidic heavy chain CDR3s that is more similar to MS patients with extensive lesion load.
DISCUSSION
We initially focused on the overall mutational frequencies and targeting of mutations to CDRs because we had observed that clonally expanded CSF B cells isolated from MS patients had high mutational frequencies and a tendancy towards diminished mutational targeting, especially as it related to RGYW/WRCY motif targeting within CDRs (Monson et al., 2005). The mutational analysis of CSF B cells isolated from eight MS, two OND and one CIS patient demonstrated that even the inclusive CSF B cell repertoires from such patients have unusually high mutational frequencies, likely reflecting the higher frequency of memory (CD27+) B cells in CSF compared to HCPB (Figure 1). Memory B cells have already been selected based on their antigen specificity, and so it is possible that even those memory B cells that we did not identify as members of a clonal population are also reactive to antigens within the CNS, and we have simply caught them at a moment in time when they have not undergone the final step of activation and differentiation into antibody secreting cells. Indeed, it has recently been reported that expansion of short-lived plasmablasts (which maintain CD19 expression) in the CSF of MS patients correlates with inflammation in MS (Cepok et al., 2005), and that the likely source of these cells is persistent memory CD19+ B cells in the CNS.
Our data suggest that the CSF B cells from MS, patients were selected in the context of germinal centers, since CDR mutation targeting characteristic of GC-derived B cells was intact in these populations (see Tables 5–7). However, our previous data had suggested that these targeting mechanisms were not consistently intact in the CSF B cell clones found in the MS patients, especially targeting to RGYW/WRCY motifs within CDRs. Table 8 provides a summary of mutational targeting of heavy chain CSF B cell clonal repertoires from MS patients which illustrates that although the majority of clones do demonstrate targeting of mutations to CDRs, targeting of these CDR mutations within RGYW/WRCY motifs was not as impressively observed as it is in the overall CSF B cell repertoires from these same patients, or in HCPB. Although targeting of mutations to RGYW/WRCY motifs within CDRs is only one measurement, it is a very well-established readout of GC-dependant mutation accumulation (Dorner et al., 1998a; Foster et al., 1999; Milstein et al., 1998; Monson et al., 2000; Monson et al., 2001; Odegard and Schatz, 2006; Oprea et al., 2001; Rogozin and Diaz, 2004; Rogozin and Kolchanov, 1992; Rogozin et al., 2001). Thus, its diminished impact on these clonally expanded CSF B cells from MS patients supports the possibility that clonal expansion of self-reactive CSF B cells may occur independent of classical germinal centers. This is not an incongruous concept, since others have reported that self-reactive B cells in SLE mouse models do expand outside the context of GCs (William et al., 2002). In addition, Corcione et al have proposed that one possible mechanism of CSF B cell repertoire generation is by activated B cells directly differentiating into short-lived plasma cells without passing through germinal centers (Corcione et al., 2005), which would parallel current opinions within the SLE community. Of note, the B cell repertoires that we studied here would have included these short-lived plasma cells, which are CD19+.
Alternatively, (and more likely) clonal expansion of CSF B cells occurred in the context of GCs, but perhaps these particular GCs do not confer the same restriction on B cell selection (including particular mutation patterns) as classical peripheral secondary lymphoid follicles confer on B cells. Serafini et al have documented that GC-like follicles can be detected in the meninges of MS patients that contain severe demyelinating lesions, and such ectopic (or non-classical) GCs may be permissive repositories for clonal expansion of autoreactive B cells in the CNS. Unfortunately, we cannot determine from this analysis whether the CSF B cells we analyzed here matriculated from follicles within the CNS, or even whether germinal centers could be detected in any of these patients. However, we can confirm that the CSF B cell repertoire in these MS patients adheres to mutation patterns consistent with GC-derived B cells, and that in some cases, clonally expanded CSF B cells from some MS patients do not adhere to mutation patterns consistent with GC-derived B cells, especially as they relate to RGYW/WRCY targeting within CDRs. Also recall that the frequency of net acidic CDR3 heavy chains from the two OND patients, one CIS patient, and the Haubold optic neuritis patientwas somewhat decreased in comparison to CDMS, and mature B cells. This observation suggests that those B cells with net acidic charges in their heavy chain CDR3s may have an advantage to enter and reside in CNS of patients with CDMS, but not in the CNS of patients who are either “preclinical” for MS, or have some other neurological disease. It is possible that these CSF B cells also have a higher propensity to bind proteins with net basic charges, but antigen-antibody interactions are more likely dependant on the position of particular acidic residues, and not on net acidic charge. Perhaps there is something different in the milieu of patients with clinically definite MS that permits enrichment of these cells in clinically definite MS patients, such as the degree of inflammation and/or lesion load within the CNS. Preventing enrichment of CSF B cells in MS patients that may be poised to recognize basic proteins prevalent in the CNS may provide an avenue to dampen potential contributions of B cells to the pathogenesis of MS.
Supplementary Material
Acknowledgments
The authors wish to thank the patients who consented to sampling for this study. Bonnie Darnell and Angie Mobley are thanked for their technical expertise in cell sorting, and Tanya Hendricks and Timothy Ahearne for repertoire analysis support. The authors would also like to thank Drs. E. Sally Ward and Petra Cravens for critical review of this manuscript.
This study was supported by grants from the National Institutes of Health (NIH) to MKR (RO1 NS 37513, RO1 AI 47133, and K24 NS 44250) and NLM (RO1 NS 40993), the National Multiple Sclerosis Society (NMSS) to NLM (RG 3267-A-1) and the Yellow Rose Foundation (MKR and NLM). NLM is a Wadsworth Foundation Young Investigator. CH was supported by NIH NRSA5 T32 AI 005284-28 from NIAID. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blalock JE, Zhou SR, Maier CC, Galin FS, Whitaker JN. Highly related immunolgobulin light chain sequences in different multiple sclerosis patients. J Neuroimmunol. 1999;100:98–101. doi: 10.1016/s0165-5728(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Brezinschek H-P, Brezinschek RI, Lipsky PE. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol. 1995;155:190–202. [PubMed] [Google Scholar]
- Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J, Sommer N, Hartung HP, Hemmer B. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- Columbo M, Dono M, Gazzola P, Roncella S, Valetto A, Chiorazzi N, Mancardi GL, Ferrarini M. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- Corcione A, Aloisi F, Serafini B, Capello E, Mancardi GL, Pistoia V, Uccelli A. B-cell differentiation in the CNS of patients with multiple sclerosis. Autoimmun Rev. 2005;4:549–554. doi: 10.1016/j.autrev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi GL, Uccelli A, Pistoia V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner T, Brezinschek H-P, Brezinschek RI, Foster SJ, Domiati-Saad R, Lipsky PE. Analysis of the frequency and pattern of somatic mutations within nonproductively rearranged human variable heavy chain genes. J Immunol. 1997;158:2779–2789. [PubMed] [Google Scholar]
- Dorner T, Brezinschek H-P, Foster SJ, Brezinschek RI, Farner NL, Lipsky PE. Comparable impact of mutational and selective influences in shaping the expressed repertoire of peripheral IgM+/CD5− and IgM+/CD5+ B cells. Eur J Immunol. 1998a;28:657–668. doi: 10.1002/(SICI)1521-4141(199802)28:02<657::AID-IMMU657>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dorner T, Brezinschek H-P, Foster SJ, Brezinschek RI, Farner NL, Lipsky PE. Delineation of selective influences shaping the mutated expressed human Ig heavy chain repertoire. J Immunol. 1998b;160:2831–2841. [PubMed] [Google Scholar]
- Dorner T, Farner NL, Lipsky PE. Two mechanisms of somatic hypermutation of Ig V genes. The Immunologist. 1999;7:153–162. [Google Scholar]
- Dorner T, Foster SJ, Farner NL, Lipsky PE. Somatic hypermutation of human immunoglobulin heavy chain genes: targeting of RGYW motifs on both DNA strands. Eur J Immunol. 1998c;28:3384–3396. doi: 10.1002/(SICI)1521-4141(199810)28:10<3384::AID-IMMU3384>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Farner NL, Dorner T, Lipsky PE. Molecular and selective mechanisms influence the distribution of VIJI rearrangements in normal human B cell. J Immunol. 1999a;162:1027–1036. [Google Scholar]
- Farner NL, Dorner T, Lipsky PE. Molecular mechanisms and selection influence the generation of the human VlJl repertoire. J Immunol. 1999b;162:2137–2145. [PubMed] [Google Scholar]
- Foster SJ, Brezinschek HP, Brezinschek RI, Lipsky PE. Molecular mechanisms and selective influences that shape the kappa chain repertoire of IgM+ B cells. J Clin Invest. 1997;99:1614–1627. doi: 10.1172/JCI119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SJ, Dorner T, Lipsky PE. Somatic hypermutation of VKJK rearrangements: targeting of RGYW motifs on both DNA strands and preferential selection of mutated codons within RGYW motifs. Eur J Immunol. 1999;29:4011–4021. doi: 10.1002/(SICI)1521-4141(199912)29:12<4011::AID-IMMU4011>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Harling-Berg C, Knopf P, Merriam J, Cserr H. Role of cervical lymph nodes in the systemic humoral immune response to human serum albumin microinfused into rat cerebrospinal fluid. J Neuroimmunol. 1989;25:185–193. doi: 10.1016/0165-5728(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Haubold K, Owens GP, Kaur P, Ritchie AM, Gilden DH, Bennett JL. B-lymphocyte and plasma cell clonal expansion in monosymptomatic optic neuritis cerebrospinal fluid. Ann Neurol. 2004;56:97–107. doi: 10.1002/ana.20152. [DOI] [PubMed] [Google Scholar]
- Hochwald G, Driel AV, Robinson M, Thorbecke G. Immune response in draining lymph nodes and spleen after intraventricular injection of antigen. Intern J Neurosci. 1988;39:299–306. doi: 10.3109/00207458808985717. [DOI] [PubMed] [Google Scholar]
- Jolly CJ, Wagner SD, Rada C, Klix N, Milstein C, Neuberger MS. The targeting of somatic hypermutation. Semin Immunol. 1996;8:159–168. doi: 10.1006/smim.1996.0020. [DOI] [PubMed] [Google Scholar]
- Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf P, Cserr H, Nolan S, Wu T, Harling-Berg C. Physiology and immunology of lymphatic drainage of interstitial and cerebrospinal fluid from the brain. Neuropathol Appl Neurobiol. 1995;21:175–180. doi: 10.1111/j.1365-2990.1995.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Knopf P, Harling-Berg C, Cserr H, Basu D, Sirulnick E, Nolan S, Park J, Keir G, Thompson E, Hickey W. Antigen dependant intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen specific B cells. J Immunol. 1998;161:692–701. [PubMed] [Google Scholar]
- Milstein C, Neuberger MS, Staden R. Both DNA strands of antibody genes are hypermutation targets. Proc Natl Acad Sci U S A. 1998;95:8791–8794. doi: 10.1073/pnas.95.15.8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson NL, Brezinschek HP, Brezinschek RI, Mobley A, Vaughan GK, Frohman EM, Racke MK, Lipsky PE. Receptor revision and atypical mutational characteristics in clonally expanded B cells from the cerebrospinal fluid of recently diagnosed multiple sclerosis patients. J Neuroimmunol. 2005;158:170–181. doi: 10.1016/j.jneuroim.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Monson NL, Dorner T, Lipsky PE. Targeting and selection of mutations in human V-lambda rearrangements. Eur J Immunol. 2000;30:1597–1605. doi: 10.1002/1521-4141(200006)30:6<1597::AID-IMMU1597>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Monson NL, Foster SJ, Brezinschek HP, Dorner T, Lipsky PE. The role of CD40-CD40 ligand (CD154) interactions in immunoglobulin light chain repertoire generation and somatic mutation. Clin Immunol. 2001;100:71–81. doi: 10.1006/clim.2001.5049. [DOI] [PubMed] [Google Scholar]
- Nemazee D. Receptor editing in B cells. Adv Immunol. 2000;74:89–126. doi: 10.1016/s0065-2776(08)60909-8. [DOI] [PubMed] [Google Scholar]
- Neuberger MS, Ehrenstein MR, Klix N, Jolly CJ, Yelamos J, Rada C, Milstein C. Monitoring and interpreting the intrinsic features of somatic hypermutation. Immunol Rev. 1998;162:107–116. doi: 10.1111/j.1600-065x.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Neuberger MS, Milstein C. Somatic hypermutation. Curr Opin Immunol. 1995;7:248–254. doi: 10.1016/0952-7915(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- Oprea M, Cowell LG, Kepler TB. The targeting of somatic hypermutation closely resembles that of meiotic mutation. J Immunol. 2001;166:892–899. doi: 10.4049/jimmunol.166.2.892. [DOI] [PubMed] [Google Scholar]
- Owens G, Ritchie A, Burgoon M, Williamson R, Corboy J, Gilden D. Single Cell Repertoire Analysis Demonstrates Clonal Expansion is Prominent Feature of the B cell Response in Multiple Sclerosis Spinal Fluid. J Immunol. 2003;171:2725–2733. doi: 10.4049/jimmunol.171.5.2725. [DOI] [PubMed] [Google Scholar]
- Phillips M, Needham M, Weller R. Role of cervical lymph nodes in autoimmune encephalomyelitis in the lewis rat. J Pathol. 1997;182:457–464. doi: 10.1002/(SICI)1096-9896(199708)182:4<457::AID-PATH870>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Prinease J. Multiple sclerosis: presence of lymphatic capillaries and lymphoid tissue in the brain and spinal chord. Science. 1979;203:1123–1125. doi: 10.1126/science.424741. [DOI] [PubMed] [Google Scholar]
- Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. Clonal expansion and somatic hypermutation of VH genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest. 1998;102:1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada C, Ehrenstein MR, Neuberger MS, Milstein C. Hot spot focusing of somatic hypermutation in MSH2-deficient mice suggests two stages of mutational targeting. Immunity. 1998;9:135–141. doi: 10.1016/s1074-7613(00)80595-6. [DOI] [PubMed] [Google Scholar]
- Ritchie AM, Gilden DH, Williamson RA, Burgoon MP, Yu X, Helm K, Corboy JR, Owens GP. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2004;173:649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Diaz M. Cutting edge: DGYW/WRCH is a better predictor of mutability at G:C bases in Ig hypermutation than the widely accepted RGYW/WRCY motif and probably reflects a two-step activation-induced cytidine deaminase-triggered process. J Immunol. 2004;172:3382–3384. doi: 10.4049/jimmunol.172.6.3382. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Kolchanov NA. Somatic hypermutagenesis in immunoglobulin genes. II. Influence of neighbouring base sequences on mutagenesis. Biochem Biophys Acta. 1992;1171:11–18. doi: 10.1016/0167-4781(92)90134-l. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Pavlov YI, Bebenek K, Matsuda T, Kunkel TA. Somatic mutation hotspots correlate with DNA polymerase eta error spectrum. Nat Immunol. 2001;2:530–536. doi: 10.1038/88732. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Jensen CV, Christiansen M. Intrathecal IgG synthesis and autoantibody secreting cells in multiple sclerosis. J Neuroimmunol. 2000;108:207–215. doi: 10.1016/s0165-5728(00)00292-7. [DOI] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, Lassmann H, Olsen DB, Strieter RM, Ransohoff RM, Sellebjerg F. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- Torcia M, Chiara G, Nencioni L, Ammendola s, Labardi d, Lucibello M, Rosini P, Marlier L, Bonini P, Sbarba P, Palamara A, Zambrano N, Russo T, Garaci E, Cozzolino F. Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation and cytochrome C release. J Biol Chem. 2001;276:39027–39036. doi: 10.1074/jbc.M102970200. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Widner H, Moller G, Johansson B. Immune response in deep cervical lymph nodes and spleen in the mouse after antigen deposition in different intracerebral sites. Scand J Immunol. 1988;28:563–571. doi: 10.1111/j.1365-3083.1988.tb01488.x. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- Yousfi M, Yousfi M, Yousfi V, Yousfi G, Yousfi D, Yousfi C, Yousfi M-P, Yousfi L. IMGT/JunctionAnalysis : the first tool for the analysis of the immunoglobulin and T cell receptor complex V-J and V-D-J JUNCTIONs. Bioinformatics. 2004;20:I379–I385. doi: 10.1093/bioinformatics/bth945. [DOI] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


