Abstract
Classical conditioning of the eyeblink reflex is elicited by paired presentation of a conditioned stimulus and an unconditioned stimulus and represents a basic form of cerebellum-dependent motor learning. Purkinje cells and the deep nuclei receive convergent information of conditioned stimulus and unconditioned stimulus through the mossy fiber and climbing fiber projections, respectively. To explore the relative importance of these neural circuits and the underlying mechanism in associative eyeblink learning, we adopted a novel gene-manipulating technique, termed reversible neurotransmission blocking (RNB). In this technology, cerebellar granule cells specifically expressed neurotransmission-blocking tetanus toxin in a doxycycline (DOX)-dependent manner. Extracellular recording of Purkinje cells in awake RNB mice revealed that DOX treatment and withdrawal reversibly turned off and on simple spikes elicited by granule cell inputs, respectively, without interference with complex spikes evoked by climbing fiber inputs. Blockade of granule cell inputs to Purkinje cells abolished eyeblink conditioned responses (CRs) in a DOX-dependent manner. Importantly, when granule cell inputs recovered by removal of DOX, normal CRs were immediately produced in the DOX-treated, CR-negative RNB mice from the beginning of reconditioning. This learning process in RNB mice during DOX treatment was completely abolished by bilateral lesion of the interpositus nucleus before eyeblink conditioning. These results indicate that the convergent information at the interpositus nucleus is critical for acquisition and storage of learning in intimate association with the Purkinje cell circuit for expression of CRs in eyeblink conditioning.
Keywords: eyeblink conditioning, interpositus nucleus, Purkinje cell, reversible neurotransmission blockade, synaptic plasticity
Classical conditioning of the eyeblink reflex is elicited by paired presentation of conditioned stimulus (CS) with reinforcing unconditioned stimulus (US) (1–4). This associative motor learning is a basic form of cerebellum-dependent leaning. In eyeblink conditioning, the CS pathway includes the pontine nucleus and mossy fibers, whereas the US pathway includes the inferior olive and the climbing fibers (1–5). The mossy fibers project directly to the interpositus nucleus and indirectly to the cerebellar cortex via granule cells (1–5). The CS and US signals are thus conveyed to both the cerebellar cortex and the interpositus nucleus (Fig. 1a). The localization and underlying mechanisms of eyeblink conditioning have been extensively studied by different approaches including gene targeting (6–11), lesioning (12–15), mutant analysis (16), and pharmacological inactivation analyses (17–23). On the basis of these studies, one model proposes an essential role of the cerebellar Purkinje cell circuit in memory traces (24–26). According to this model, convergence of the CS and US signals induces long-term depression at the parallel fiber–Purkinje cell synapses. Because Purkinje cells are inhibitory, long-term depression causes a disinhibition of the interpositus nucleus and an increased input in the downstream motor pathways. The other model proposes that the memory trace is located in the interpositus nucleus and that conditioned responses (CRs) are properly evoked in response to CS by a timing signal from Purkinje cells (1, 3, 27, 28). Eyeblink conditioning involves multiple learning processes, at least acquisition, expression, and storage of motor learning. Once the expression process of memory traces is impaired, it becomes difficult to define the key neural circuits responsible for expression, formation, and storage of memory traces.
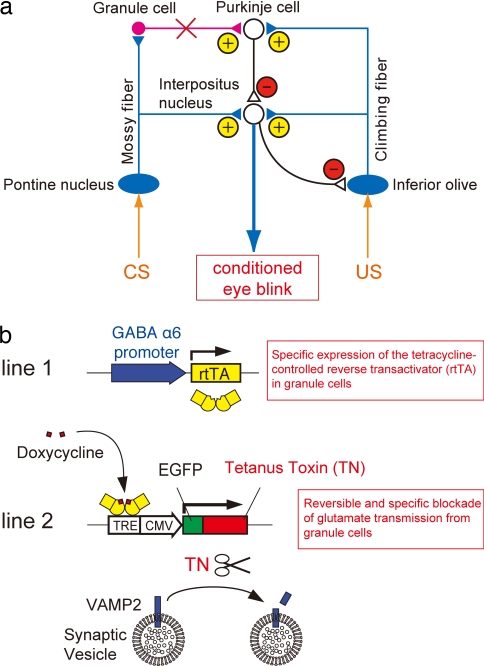
Fig. 1.
Scheme of the cerebellar circuitry and the RNB technique. (a) The mossy fibers project directly to the interpositus nucleus and indirectly to Purkinje cells. The climbing fibers project to both Purkinje cells and the interpositus nucleus. Purkinje cells are the sole inhibitory output system from the cerebellar cortex. Feedback projections of several inhibitory interneurons are omitted in this diagram. Red X, blockade of granule cell transmission; yellow plus signs, excitatory; red minus signs, inhibitory. (b) In RNB mice, the expression of rtTA is confined to granule cells by the granule cell-specific GABAA receptor α6-subunit promoter. DOX-bound rtTA induces restrictedly the expression of tetanus toxin in granule cells, which in turn cleaves VAMP2 and blocks glutamatergic transmission. TRE, tetracycline-responsive element; CMV, cytomegalovirus promoter.
In this investigation, we adopted a novel reversible neurotransmission blocking (RNB) technique to explore the role of Purkinje cells and the interpositus nucleus in associative eyeblink conditioning. In the RNB transgenic mice, the tetanus toxin light chain is restrictedly expressed in granule cells under the control of a tetracycline-controlled reverse transactivator (rtTA) (29). Tetanus toxin specifically cleaves synaptic vesicle VAMP2 (30), resulting in blockade of transmitter release from synaptic vesicles (Fig. 1b) (29). Consequently, granule cell inputs to Purkinje cells are blocked and reversibly recovered by administration and omission, respectively, of doxycycline (DOX) (29). These RNB mice were thus used based on the following rationale: when granule cell inputs to Purkinje cells are selectively blocked, the CS signal is not transmitted to Purkinje cells but still conveyed to the interpositus nucleus via the direct mossy fiber pathway (Fig. 1a). The reversible blockade of granule cell transmission can thus delineate distinct roles of the direct mossy fiber-mediated information and the indirect granule cell-mediated information in acquisition, expression, and storage of CRs in associative eyeblink conditioning.
Results
Specific and Reversible Blockade of Granule Cell Transmission.
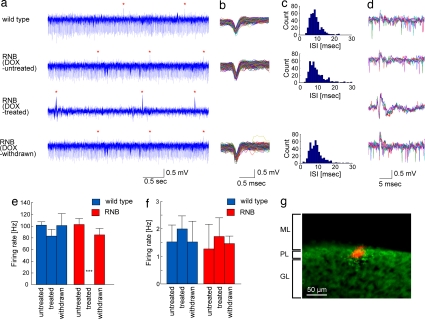
Our previous study showed that tetanus toxin was fully induced in RNB mice 5 days after DOX treatment and remained at maximal levels thereafter (29). This study showed that the tetanus toxin expression completely disappeared in RNB mice when DOX was withdrawn for 14 days and that the toxin was restrictedly expressed in granule cells and specifically cleaved VAMP2 among neuronal proteins. In addition, the DOX-treated RNB mice showed no abnormal motor behaviors such as ataxia or tremor under the ordinary condition, nor was there any alteration in the cerebellar architecture with respect to the cell number, shape, or anatomical arrangement after DOX treatment of the RNB mice. Our previous study also showed that KCl-evoked depolarization greatly reduced but did not completely prevent glutamate release in cerebellar slices of DOX-treated RNB mice (29). This residual release was most likely due to concomitantly enhanced glutamate release from other cerebellar cell types. We therefore more precisely examined how efficiently and selectively DOX treatment blocks granule cell transmission by electrophysiological recording (31). WT and RNB mice were treated or not with DOX for 14 days. We then performed extracellular recording of Purkinje cells at the simplex lobe of the cerebellum of awake animals, which area has been implicated in eyeblink CRs (3). After every recording, the recording site at the Purkinje cell layer was confirmed by the injection of fluorescent dye from the recording electrode (Fig. 2g). In WT mice, relatively regular firing of simple spikes and occasional complex spikes was evoked by neurotransmissions from granule cells and climbing fibers to Purkinje cells, respectively (Fig. 2a). The DOX-untreated RNB mice comparably exhibited both simple spikes and complex spikes (Fig. 2 a, e, and f). Remarkably, simple spikes were completely abolished in DOX-treated RNB mice [P < 0.001, cells of DOX-treated (n = 6) vs. DOX-untreated (n = 6) mice] (Fig. 2 a and e). When DOX was withdrawn for 14 days after 14-day DOX treatment, these simple spikes reversibly recovered to their normal levels in DOX-withdrawn mice (P > 0.99) (Fig. 2 a and e). Importantly, complex spikes remained unchanged in RNB mice, irrespective of administration and omission of DOX [P > 0.99, cells of WT (n = 7) vs. RNB (n = 6) mice] (Fig. 2 a and f). There was no difference in the firing patterns and frequencies of either simple spikes or complex spikes or in the peaks of interspike intervals of simple spikes between WT mice (7.4 ± 0.4 msec; cells, n = 7) and DOX-untreated (8.2 ± 0.7 msec; cells, n = 6)/DOX-withdrawn (10.2 ± 1.2 msec; cells, n = 5) RNB mice (P > 0.82) (Fig. 2 b–f). Furthermore, simple spikes were never observed even when a tone stimulus was given to DOX-treated RNB mice (data not shown). The results indicate that the RNB mice reversibly lost granule cell inputs to Purkinje cells in a DOX-dependent manner without interference with the responsiveness of Purkinje cells to climbing fiber inputs.
Fig. 2.
Action potential firing of Purkinje cells in awake WT and RNB transgenic mice. (a) Spontaneous activities of Purkinje cells consisted of simple spikes and complex spikes (red asterisks) in WT, DOX-untreated, and DOX-withdrawn RNB mice, but no simple spikes were evoked in DOX-treated RNB mice. (b) Traces of simple spikes shown in a were superimposed. (c) Interspike interval histogram of simple spikes shown in b. (d) Traces of five complex spikes were superimposed. (e and f) Mean ± SEM of firing rates of simple spikes (e) and complex spikes (f) are shown with columns and bars, respectively. ***, P < 0.001 (comparison between DOX-treated RNB mice and all five other animals by the Scheffé test). (g) The extracellular recording site (red) was confirmed at the Purkinje cell layer by fluorescent Nissl staining. ML, molecular layer; PL, Purkinje cell layer; GL, granule cell layer.
Effects of Reversible Blockade of Granule Cell Transmission on Eyeblink Conditioning.
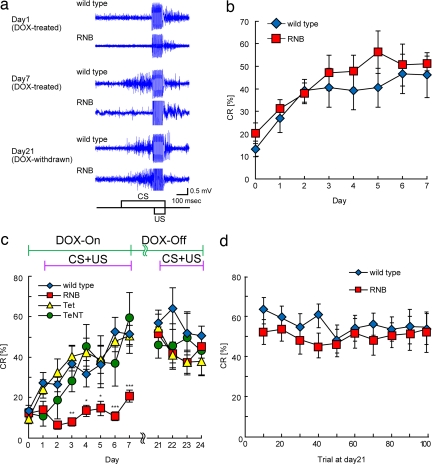
We examined how reversible blockade of granule cell transmission influences the acquisition, expression, and storage of associative eyeblink conditioning. We first tested the effect of blocking granule cell transmission on the execution of eyeblink conditioning. Eyeblink conditioning comprised seven daily sessions in which a tone was paired with a periorbital electrical US. Animals were pretreated with DOX for 14 days. Eyeblink conditioning and CR measurement were then performed from day 1 to day 7 during the continuous administration of DOX (the first conditioning). WT mice showed a gradually increasing and significant number of CRs during eyeblink conditioning (n = 9, session, F = 479, P < 0.0001) (Fig. 3 a and b). Similarly, the RNB mice, when not treated with DOX, showed a comparable increase in the number of CRs during the 7-day conditioning (n = 8, session, F = 414, P < 0.0001) (Fig. 3b). In marked contrast, DOX-treated RNB mice failed to evoke CRs during the 7 days of conditioning, and the difference from WT mice was significant (RNB, n = 12; WT, n = 6; genotype, F = 19.4, P < 0.001; session and genotype interaction F = 4.36, P < 0.01) [Fig. 3 a and c and supporting information (SI) Fig. 5]. These results indicate that the CS signal to Purkinje cells is indispensable for learned responses in eyeblink conditioning.
Fig. 3.
Conditioned eyeblink responses. (a) The representative EMG amplitudes of CRs of WT and RNB mice on days 1, 7, and 21 are indicated. (b and c) Data of CRs of DOX-untreated WT and RNB mice (b) and those of CRs of four lines of mice with DOX treatment and withdrawal (c) are expressed as percent CRs averaged over all animals in each group for each conditioning day. In b, n = 9 (WT) and 8 (RNB); in c, n = 6 (WT), 12 (RNB), 11 (Tet), and 6 (TeNT). Data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (DOX-treated WT mice vs. DOX-treated RNB mice). (d) Mean ± SEM of percent CRs for DOX-withdrawn WT and RNB mice as a function of conditioning block on day 21. Each block consisted of nine paired CS–US trials and one CS only at the 10th trial.
We then tested whether the RNB mice form any learning processes during DOX treatment. DOX was withdrawn from DOX-treated, conditioned RNB mice from day 8 to day 24. CRs were then measured on the last 4 consecutive days by the second conditioning (Fig. 3 a and c and SI Fig. 5). The learned WT mice showed significant CRs from the beginning of the second conditioning (P < 0.0001, day 1 vs. day 21). Importantly, the DOX-withdrawn RNB mice exhibited comparable levels of CRs when granule cell inputs to Purkinje cells fully recovered by the removal of DOX (genotype, F = 0.086, P > 0.77; session, F = 1.75, P > 0.18; genotype and session interaction, F = 0.249, P > 0.86). This was evident from a block-by-block analysis of DOX-withdrawn RNB mice, indicating a normal performance of CRs throughout the course of a single conditioning session on day 21 (Fig. 3d) (genotype, F = 0.726, P > 0.40; block and genotype interaction, F = 0.312, P > 0.97). Moreover, the DOX-withdrawn RNB mice, like WT mice, showed well timed onset and peak of the CRs relative to US in the second conditioning (Fig. 3a). In the experiment shown in Fig. 3c, we also examined two additional transgenic lines as controls, one (TeNT mouse) encoding the tetanus toxin gene alone and the other (Tet mouse) encoding the rtTA gene alone. There was no difference in the ability to evoke CRs between the WT mice and these two transgenic lines (Fig. 3c) (TeNT, n = 6; Tet, n = 11; the first conditioning, genotype, F = 0.445, P > 0.64, and the second conditioning, genotype, F = 0.369, P > 0.69). Furthermore, the reversibility of CR blockade was reproduced in RNB transgenic mice by repeated cycles of DOX treatment and withdrawal (data not shown). The results thus indicate that the failure of DOX-treated RNB mice to express CRs depends on the expression of tetanus toxin that results in blockade of granule cell transmission to Purkinje cells. Collectively, these results explicitly demonstrate that the memory of eyeblink CRs is acquired and saved despite the absence of the CS signals to Purkinje cells and also indicate that the normal granule cell transmission is necessary for expression of the stored memory of eyeblink conditioning.
Abolition of CRs by Bilateral Lesioning of the Interpositus Nucleus.
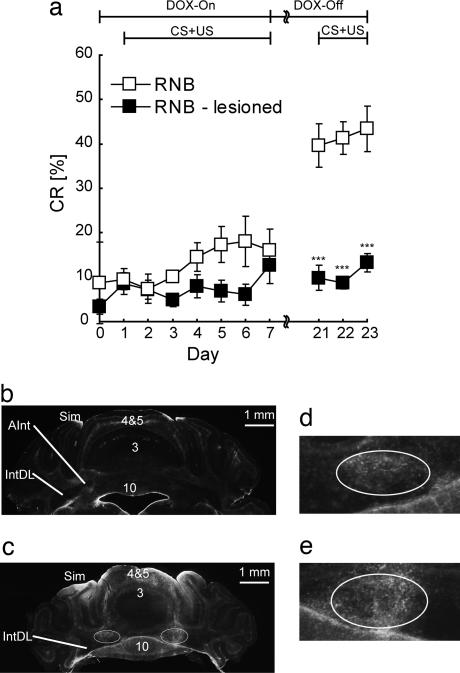
To address whether the latent CRs are formed in the cerebellar deep nuclei of DOX-treated RNB mice, we lesioned the interpositus nucleus electrolytically or not (sham-operated) and 7 days later performed the CR analysis according to the same procedure as described in Fig. 3. DOX-withdrawn RNB mice with their interpositus nucleus bilaterally lesioned, unlike sham-operated mice, showed loss of CRs at the second conditioning (Fig. 4a) (operation, F = 40.2, P < 0.0001; session, F = 1.91, P > 0.16). Upon coronal section analysis, lesions were confirmed to be located at the anterior interpositus nucleus region critical for eyeblink CRs (Fig. 4 b–e). These results thus indicate that the interpositus nucleus is essential for the latent acquisition and storage of CRs during blockade of granule cell inputs to Purkinje cells.
Fig. 4.
Abolition of CRs by bilateral interpositus nucleus lesion. Seven lesioned and seven sham-operated animals were confirmed to be lesioned and intact in the interpositus nucleus after histological analysis and were used for data analysis. (a) Data are presented as the mean ± SEM. ***, P < 0.001 (sham-operated vs. lesioned). (b–e) Histological analysis of bilateral interpositus nucleus lesions. Typical examples of coronal sections stained with anti-glial fibrillary acidic protein antibody from sham-operated (b) and lesioned (c) RNB mice are shown. Enclosures of the left and right interpositus nucleus regions in c are expanded in d and e, respectively, indicating gliosis in the interpositus nucleus region ≈30 days after electrolytic lesioning. Sim, simplex lobe; 3, 4, 5, and 10, lobe numbers; IntDL, dorsolateral interpositus nucleus; AInt, anterior interpositus nucleus.
Discussion
The present investigation indicates that the reversible expression of tetanus toxin in granule cells turned on and off the mossy fiber inputs to Purkinje cells without interfering with inputs to the interpositus nucleus. This blockade of granule cell inputs abolished expression of eyeblink CRs in a DOX-dependent manner. Remarkably, when granule cell inputs to Purkinje cells recovered, once conditioned, CR-negative RNB mice immediately evoked normal CRs from the beginning of the second conditioning. This learned process required the intact circuit of the interpositus nucleus. The present investigation thus demonstrates that the basic memory trace is formed and stored by convergent information of CS and US at the interpositus nucleus and that the expression of this memory requires the transmission of the CS and US signals at the Purkinje cell circuit in eyeblink conditioning.
Previous electrophysiological recording in isolated/slice preparations or in anesthetized animals indicated that Purkinje cells fire spontaneously in the absence of synaptic input (32–34). In contrast to these findings, blockade of granule cell transmission abolished firing of simple spikes in the DOX-treated awake animals. In the cerebellar cortical circuitry, basket, stellate, and Golgi interneurons receive granule cell transmission and could influence the responsiveness of Purkinje cells (5). However, these interneurons are all inhibitory and would facilitate firing of simple spikes by blockade of granule cell transmission. No such facilitation of simple spike firing was observed in the DOX-treated RNB mice. The cerebellar nuclei also send inhibitory projections to the inferior olive (Fig. 1a). It is thus possible that blockade of granule cell transmission leads to a reduction in inhibition in the cerebellar nuclei, which could in turn result in inhibition in the inferior olive via the nuclear–olivary inhibitory pathway (35). However, we observed no change in either the pattern or the frequency of complex spikes elicited by the climbing fiber input. Furthermore, there was no difference in responsiveness of electromyogram (EMG) to US between DOX-treated RNB mice and WT mice (Fig. 3a). Although the difference in the firing mechanism of Purkinje cells between this study and other studies remains elusive, the present investigation indicates that the CS signal to Purkinje cells is reversibly blocked in a DOX-dependent manner without interfering with the US signal.
Accumulated evidence has indicated that both Purkinje cells and interpositus nucleus circuits play a critical role in the execution and proper timing of CRs in associative eyeblink motor learning (6–23). The CS and US signals are conveyed to both the Purkinje cell and the interpositus nucleus circuits, and the convergent information of these two signals is capable of inducing neural plasticity at both circuits (2, 26, 36, 37). In the Purkinje cell circuit, long-term depression is induced at the parallel fiber–Purkinje cell synapses by the conjunctive stimulation of parallel fibers and climbing fibers and suppresses the tonic inhibition of Purkinje cells in the interpositus nucleus circuit. Long-term depression thus causes an increased CS input in the downstream motor pathways. In this investigation, the reversible blocking manipulation explicitly demonstrates that the memory trace of CRs is acquired and stored in the interpositus nucleus in the absence of granule cell transmission to Purkinje cells. The critical role of the interpositus nucleus for acquisition and storage of learning is consistent with many other studies (refs. 3, 12, 13, 38, and 39, but see also refs. 22 and 24). In DOX-treated RNB mice, blockade of granule cell transmission relieves the tonic Purkinje cell inhibition, and the interpositus nucleus circuit would induce neural plasticity in response to the convergent information of the CS and US signals. This neural plasticity could thus allow prompt induction of CRs to the coincident CS and US information once granule cell transmission recovers by removal of DOX. It should, however, be pointed out that lesioning of the cerebellar cortex and mutation of Purkinje cell degeneration still evoked eyeblink CRs, although this response was low and had a short latency with respect to CS (15, 16). In DOX-treated RNB mice, climbing fiber inputs remain intact. Furthermore, the frequency of complex spikes has been reported to be enhanced by US (40). The climbing fiber inputs could thus allow Purkinje cells to partially suppress the interpositus nucleus activity and abolish expression of CRs in DOX-treated RNB mice. In this context, it has been reported that expression of CRs requires certain levels of depolarization of the interpositus nucleus neurons before conditioning-induced disinhibition of Purkinje cell inputs (21, 41). The expression of CRs could thus be more sensitive to the Purkinje cell-mediated inhibition in the interpositus nucleus than the acquisition and storage of CRs. Furthermore, multiple synaptic plasticity has been reported to occur at different local circuits in the cerebellum (42). The neural plasticity in these synapses could contribute to prompt induction of CRs to the coincident CS and US information once granule cell transmission recovers by removal of DOX. Therefore, it is likely that the associative eyeblink learning involves an elaborate and integrative plasticity mechanism.
Materials and Methods
Animals.
RNB mice and their WT littermates were obtained by mating TeNT and Tet transgenic mice (29) and were used for all experiments unless otherwise stated. DOX was administered in pellets containing 6 mg/g DOX and in drinking water containing 2 mg/ml DOX and 10% sucrose (29). After electrophysiological and behavioral analyses, the genotypes of the mice were determined by using tail biopsies and Southern blotting. The analyses were performed by operators who were blind to the genotype of the mice. All animal experiments were approved by the animal committees under the guidelines of Osaka Bioscience Institute and Kyoto University.
Single-Unit Recording.
Surgery and recording were performed according to the procedures described previously (31). Under ketamine and xylazine anesthesia, a head holder was fixed to the skull of a mouse and a small hole (<1.0 mm) was drilled in the occipital bone above the simplex lobe (6.0 mm posterior and 2.0 mm lateral from bregma). Recording was conducted in awake animals with a glass micropipette filled with 2 M NaCl (3–5 M Ω). The electrode tip was moved downward by using a micromanipulator. The signal was recorded, amplified, low-pass-filtered at 10 kHz, high-pass-filtered at 100 Hz, and analyzed offline with the MatLab system (MathWorks, Natick, MA). The sampling rate for analysis was 20 kHz. Simple spikes were identified by their amplitude, and complex spikes were identified visually by their characteristic wave form. The recording site was identified by electrophoresis of Alexa Fluor 594 (Invitrogen, Carlsbad, CA) followed by fluorescent Nissl staining of cerebellar sections.
Eyeblink Conditioning.
Surgery and eyeblink conditioning were conducted according to the procedure described previously (11). Briefly, two of the four wires were used to record EMG in the orbicuralis oculi muscle, and the remaining two were used to deliver electrical shocks. A tone with 452-msec duration (1 kHz, 85 dB) was used as a CS, and then an electrical shock of 100-msec duration (100-Hz square pulses) was given as an US. The US intensity was determined as the minimal current amplitude required for eliciting a constant amplitude of unconditioned eyeblink responses. A daily conditioning consisted of 100 trials grouped in 10 blocks. Each block included nine CS–US paired trials and one CS alone at the 10th trial. Data analysis of CRs was conducted according to the procedure described previously (11). The recorded EMG signal was band-pass-filtered between 0.15 and 1.0 kHz and sampled at 10 kHz by using NI-DAQ (National Instruments, Austin, TX) by MatLab software. The maximum amplitude of EMG signals during the time period of t ± 1 msec was denoted as the EMG amplitude at t. The EMG amplitude was thus a function of time and trial number. EMG amplitude data for the 300-msec period before CS onset during 100 trials were then averaged over time and trials, and SD was calculated. The average value plus the SD value was defined as the threshold. In each trial, EMG amplitude data for the 300-msec period before CS onset above the threshold were averaged over time and termed the Pre value. In the same way, the Startle value was calculated for a 30-msec period after the CS onset, and the CR value was calculated for the period of 2–202 msec before the US onset in CS–US paired trails. The time window was subsequently extended by 102 msec for the CR value calculation in the CS-only trials. If the Pre value exceeded 1% of threshold or the Startle value exceeded 30% of threshold, the trial was excluded. If the CR value exceeded 1% of threshold and exceeded twice the Pre value, it was regarded as a successful CR trial. The ratio of successful CR to valid trials was calculated and denoted as a percentage (CR%), which was presented as the mean ± SEM.
Lesion Analysis.
The mice received bilateral electrolytic lesions in the interpositus nucleus (5.9 mm posterior, 1.5 mm lateral, and 3.0 mm ventral from bregma; 1.0-mA anodal current for 10 sec) and had 7 days of recovery before eyeblink conditioning. After the behavioral analysis, anesthetized mice were perfused with PBS followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The brains were sectioned (60 μm) and stained with anti-glial fibrillary acidic protein.
Statistical Analysis.
Analysis of interspike intervals and firing rates was carried out by one-way ANOVA followed by the Scheffé post hoc test. Analysis of eyeblink CRs was performed by a two-way or repeated ANOVA. A post hoc comparison was made with the Scheffé test.
Supplementary Material
Acknowledgments
We thank Takashi Yoshida for technical advice and Kumlesh K. Dev and Larry Frye for careful reading of the manuscript. This work was supported by a Grant-in-Aid for Specially Promoted Research (17002016) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. N.W. is a fellow of the Japan Society for the Promotion of Science.
Abbreviations
- CR
conditioned response
- CS
conditioned stimulus
- US
unconditioned stimulus
- RNB
reversible neurotransmission blocking
- DOX
doxycycline
- EMG
electromyogram.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708165104/DC1.
References
- 1.Mauk MD. Neuron. 1997;18:343–346. doi: 10.1016/s0896-6273(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 2.Ito M. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 3.Christian KM, Thompson RF. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 4.De Zeeuw CI, Yeo CH. Curr Opin Neurobiol. 2005;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Llinas R, Walton K. In: The Synaptic Organization of the Brain. Shepherd GM, editor. Oxford: Oxford Univ Press; 1998. pp. 255–288. [Google Scholar]
- 6.Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 7.Miyata M, Kim HT, Hashimoto K, Lee TK, Cho SY, Jiang H, Wu Y, Jun K, Wu D, Kano M, et al. Eur J Neurosci. 2001;13:1945–1954. doi: 10.1046/j.0953-816x.2001.01570.x. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto Y, Fujimichi R, Araishi K, Kawahara S, Kano M, Aiba A, Kirino Y. Eur J Neurosci. 2002;16:2416–2424. doi: 10.1046/j.1460-9568.2002.02407.x. [DOI] [PubMed] [Google Scholar]
- 9.Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, De Zeeuw CI. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- 10.Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, et al. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto Y, Kano M. J Neurosci. 2006;26:8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick DA, Clark GA, Lavond DG, Thompson RF. Proc Natl Acad Sci USA. 1982;79:2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo CH, Hardiman MJ, Glickstein M. Exp Brain Res. 1985;60:87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- 14.Welsh JP, Harvey JA. J Neurosci. 1989;9:299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrett SP, Ruiz BP, Mauk MD. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Bao S, Lockard JM, Kim JK, Thompson RF. J Neurosci. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krupa DJ, Thompson JK, Thompson RF. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 18.Welsh JP, Harvey JA. J Physiol. 1991;444:459–480. doi: 10.1113/jphysiol.1991.sp018888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krupa DJ, Thompson RF. Learn Mem. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- 20.Attwell PJ, Rahman S, Ivarsson M, Yeo CH. J Neurosci. 1999;19:RC45. doi: 10.1523/JNEUROSCI.19-24-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao S, Chen L, Kim JJ, Thompson RF. Proc Natl Acad Sci USA. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attwell PJ, Cooke SF, Yeo CH. Neuron. 2002;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 23.Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. J Neurosci. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo CH, Hesslow G. Trends Cognit Sci. 1998;2:322–330. doi: 10.1016/s1364-6613(98)01219-4. [DOI] [PubMed] [Google Scholar]
- 25.Ito M. Brain Res. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- 26.Jirenhed DA, Bengtsson F, Hesslow G. J Neurosci. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavond DG. Ann NY Acad Sci. 2002;978:93–105. doi: 10.1111/j.1749-6632.2002.tb07558.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RF. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S. J Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, Katoh A, Ohtsuki G, Mishina M, Hirano T. J Neurosci. 2004;24:2440–2448. doi: 10.1523/JNEUROSCI.0783-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Häusser M, Clark BA. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 33.Nam SC, Hockberger PE. J Neurobiol. 1997;33:18–32. doi: 10.1002/(sici)1097-4695(199707)33:1<18::aid-neu3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Cerminara NL, Rawson JA. J Neurosci. 2004;24:4510–4517. doi: 10.1523/JNEUROSCI.4530-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang EJ. J Neurophysiol. 2002;87:1993–2008. doi: 10.1152/jn.00477.2001. [DOI] [PubMed] [Google Scholar]
- 36.Pugh JR, Raman IM. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Aizenman CD, Manis PB, Linden DJ. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 38.McCormick DA, Thompson RF. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Bao S, Thompson RF. Behav Neurosci. 1999;113:204–210. doi: 10.1037//0735-7044.113.1.204. [DOI] [PubMed] [Google Scholar]
- 40.Green JT, Steinmetz JE. Learn Mem. 2005;12:260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina JF, Mauk MD. Nat Neurosci. 2000;3(Suppl):1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- 42.Hansel C, Linden DJ, D'Angelo E. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.