Abstract
Calcitonin gene-related peptide (CGRP) is thought to be a prominent neuropeptide in cardiovascular regulation and neuroimmune modulation. There are two isoforms of CGRP (αCGRP and βCGRP), and the main CGRP receptors are probably composed of a calcitonin receptor-like receptor (CLR) and a receptor activity-modifying protein (RAMP)1. However, the physiological functions of CGRP that are mediated through the CLR/RAMP1 receptors remain to be clarified. For an improved understanding of the functions, we generated mice deficient in RAMP1, a specific subunit of CGRP receptors, by a conditional gene-targeting technique. The RAMP1-deficient mice (RAMP1−/−) exhibited high blood pressure, with no changes in heart rate. αCGRP was found to have a potent vascular relaxant activity compared with βCGRP in the artery of the WT (RAMP1+/+) mice. The activities of both CGRP isoforms were remarkably suppressed in the arteries of the RAMP1−/− mice. The LPS-induced inflammatory responses of the RAMP1−/− mice revealed a transient and significant increase in the serum CGRP levels and high serum levels of proinflammatory cytokines compared with the RAMP1+/+ mice. αCGRP and βCGRP equally suppressed the production of TNF-α and IL-12 in bone marrow-derived dendritic cells stimulated with lipopolysaccharide. Their inhibitory effects were not observed in the bone marrow-derived dendritic cells of the RAMP1−/− mice. These results indicate that CGRP signaling through CLR/RAMP1 receptors plays a crucial role in the regulation of both blood pressure by vascular relaxation and proinflammatory cytokine production from dendritic cells.
Keywords: gene-disrupted mice, calcitonin gene-related peptide, adrenomedullin, neuropeptide, dendritic cells
Calcitonin gene-related peptide (CGRP) is a 37-aa neuropeptide that is produced in the neural body of dorsal root ganglion cells and released from sensory nerve endings. There are two isoforms of CGRP: α and β in rats and mice and I and II in humans. These differ in their peptide sequences by 1 aa (rats) and 3 aa (mice and humans) of the 37 aa (1). αCGRP is produced mainly in the nervous system by the tissue-specific alternative splicing of the primary RNA transcript of the calcitonin/CGRP gene. On the other hand, βCGRP is produced not only in the neuronal tissues but also in the enteric nerves of the intestine (2) and in immune cells such as T cells (3). Pharmacologically, αCGRP is known to have the most potent vasodilatory activity (4). Most blood vessels are surrounded by a dense perivascular CGRPergic neural network, suggesting the physiological importance of CGRP in vasodilatory regulation (5). αCGRP also contributes to local neurogenic inflammation and nociception (6). Moreover, the functions of immune cells such as macrophages (7, 8), Langerhans cells (8), and T cells (9, 10) are modulated by αCGRP. However, the precise functional differences between αCGRP and βCGRP remain unclear.
Historically, CGRP receptors have been classified into two classes: CGRP1 and CGRP2 receptors. The CGRP1 receptors are more sensitive than the CGRP2 receptors to a CGRP antagonist, namely, CGRP8–37 (11, 12). On the other hand, the linear CGRP analog [Cys(ACM)2,7]-hCGRP preferentially activates the CGRP2 receptors. In recent years, the CGRP receptor has been cloned as a heterodimer of a seven-transmembrane G protein-coupled receptor called the calcitonin receptor-like receptor (CLR) and a single membrane-spanning protein called the receptor activity-modifying protein (RAMP)1 (13, 14). CLR is a common receptor subunit for both CGRP and adrenomedullin (AM), a 52-aa peptide that shares homology with CGRP to a certain extent (15). The specificity of the CGRP receptor appears to be decided by RAMP1 because the N terminus of RAMP1 is essential for CGRP binding (16). RAMP1, along with RAMP2 and RAMP3, comprises the molecules of the RAMP family. The three RAMPs share only 30% sequence identity and differ in their tissue distributions (17). Transfection experiments indicate that both RAMP2 and RAMP3, in combination with CLR, preferentially bind AM, whereas CLR/RAMP3 receptors have a weak affinity for CGRP. Analyses using the CGRP agonists and antagonists suggest that the biochemical properties of the heterodimerization of CLR and RAMP1 in the recombinant system are similar to those of the CGRP1 receptors in the tissues and cell lines (18). However, the functions of CGRP mediated through CLR/RAMP3 and the existence of CGRP2 receptors remain to be clarified.
To elucidate the physiological functions of CGRP mediated through the CLR/RAMP1 receptors in vivo, we generated RAMP1-deficient mice. We discovered that RAMP1−/− mice exhibited potent hypertension, whereas their heart rate (HR) was unaffected. The hypertension resulted mainly from the vasodilatory disorder caused by deficient αCGRP signal transduction. AM was observed to partially exert vasodilatory activity through the CLR/RAMP1 receptors. The more interesting findings were that RAMP1−/− mice exhibited a significant increase in serum CGRP levels and markedly higher serum levels of proinflammatory cytokines after LPS administration, compared with the RAMP1+/+ mice. The dysregulation of the proinflammatory cytokine levels in the RAMP1−/− mice could be attributed to the deficiency in the signal transduction of CGRP in dendritic cells (DCs). These results indicate that CGRP transduced physiologically important signals in the regulation of not only blood pressure but also the inflammatory response via CLR/RAMP1 receptors.
Results
Generation of RAMP1-Deficient Mice.
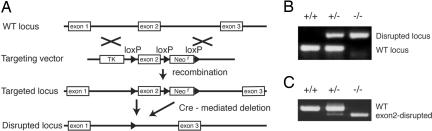
To elucidate the physiological functions of CGRP, we generated a mouse line lacking the CGRP-specific receptor subunit RAMP1. Our strategy to generate RAMP1-deficient mice was based on the utilization of the Cre–loxP system to disrupt the RAMP1 gene to determine the functions of RAMP1 in individual tissues or organs and to avoid early embryonic lethality. To generate a targeting construct, one loxP site was inserted into intron 1, and two additional loxP sites flanking Neor [the neomycin resistance gene from pPNT (19)] were placed downstream of exon 2 (Fig. 1A). Concomitant with the expression of the Cre recombinase, exon 2 of RAMP1, together with Neor, was expected to be deleted. Upon the deletion of exon 2, the splicing of exons 1 to 3 created a frameshift that resulted in the introduction of a termination codon at the beginning of exon 3. The targeting construct was transfected into D3 ES cells. Of the 643 colonies obtained after G418 and ganciclovir selection, 17 PCR-positive clones were identified. Eleven of these PCR-positive clones did not retain the 5′-loxP site within intron 1. Five of the clones bearing a 5′-loxP site contained the correctly altered RAMP1 locus as confirmed by Southern blotting, and two clones exhibited the normal karyotype (data not shown). These two RAMP1-floxed ES clones were used to generate chimeric mice, and these mice successfully contributed to the germline transmission. Furthermore, we crossbred the flox mice with CAG–Cre transgenic mice that expressed Cre recombinase in a ubiquitous manner under the transcriptional control of the chicken actin promoter. The genotypes were confirmed by PCR analysis of DNA extracted from the tail, as depicted in Fig. 1B. RT-PCR analysis indicated that the mRNA derived from exon 2 was completely missing in the thymus of the RAMP1−/− mice (Fig. 1C). The RAMP1−/− mice were born at the expected Mendelian ratios and presented no obvious abnormalities in their appearances.
Fig. 1.
Generation of RAMP1−/− mice. (A) Schematic representation of the genomic structure of the relevant part of the RAMP1 WT allele; the targeting construct with PGK–TK and mouse PGK–Neor as negative and positive selectable markers, respectively; the allele after homologous recombination; and the disrupted RAMP1 allele after Cre-mediated deletion. (B) Genotyping of mouse tail DNA by using PCR analysis involving the primers described in Materials and Methods. The WT and mutant alleles have been shown as the 108- and 240-bp fragments, respectively. (C) RT-PCR analysis of RAMP1 transcripts obtained from the thymus. Total RNA isolated from the thymus was reverse transcribed and amplified by using RAMP1-specific primer pairs.
Complete Deletion of Vasodilatory Activity of CGRP in the RAMP1−/− mice.
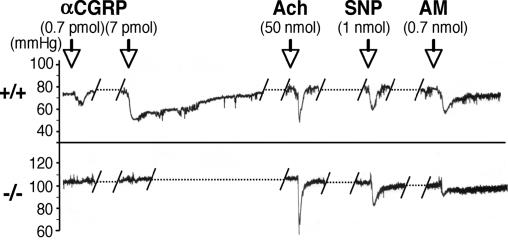
Pharmacologically, αCGRP is known to have a potent vasodilatory activity. To confirm the successful deletion of functional CGRP receptors in RAMP1−/− mice, we analyzed the fluctuation of blood pressure by administering certain vasodilators to the mice (Fig. 2). In the RAMP1+/+ mice, i.v. administration of 0.7 pmol of αCGRP induced an obvious decrease in the blood pressure due to its vasodilatory activity. In contrast, such a decrease in blood pressure was not observed in the RAMP1−/− mice administered with αCGRP at a dose of either 0.7 or 7 pmol. Both RAMP1−/− and RAMP1+/+ mice, however, exhibited normal vasodilatory responses to the injections of acetylcholine and sodium nitroprusside. These results indicate that the lack of response to the administered αCGRP in RAMP1−/− mice was not due to the dysfunction of smooth muscle cells or endothelial cells in the blood vessel but to the disappearance of the functional CGRP receptors. Interestingly, the RAMP1−/− mice exhibited slight but obvious suppression of the decrease in the blood pressure on AM administration (0.7 nmol) compared with the RAMP1+/+ mice, suggesting that AM might partially transduce the vasodilatory signal through the CLR/RAMP1 receptors in the artery.
Fig. 2.
Typical recordings showing changes in blood pressure upon injecting CGRP, acetylcholine (Ach), sodium nitroprusside (SNP), and AM in the RAMP1+/+ and RAMP1−/− mice. αCGRP (0.7 and 7 pmol/0.1 ml per mouse, i.v.), acetylcholine (50 nmol/0.1 ml per mouse, i.v.), sodium nitroprusside (1 nmol/0.1 ml per mouse, i.v.), and AM (0.7 nmol/0.1 ml per mouse, i.v.) were administered to the RAMP1+/+ (upper trace) and RAMP1−/− (lower trace) mice.
RAMP1−/− Mice Exhibit High Blood Pressure.
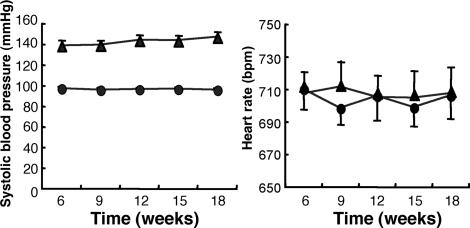
In the experiments described above, we noticed that the basal blood pressure of the RAMP1−/− mice was higher than that of the RAMP1+/+ mice. Therefore, we examined the systolic blood pressure (SBP) and HR in RAMP1+/+ and RAMP1−/− mice between 6 and 18 weeks of age under conscious conditions. As shown in Fig. 3A, at 6 weeks of age, the RAMP1−/− mice demonstrated a significantly higher SBP than the RAMP1+/+ mice (138.6 ± 2.6 vs. 97.3 ± 1.7 mmHg; P < 0.001). The SBP of the RAMP1−/− mice exhibited a progressive increase depending on the age of mice, reaching a value of 146.8 ± 2.4 mmHg at 18 weeks of age. By contrast, the HR values were indistinguishable between the RAMP1+/+ and RAMP1−/− mice at any age (Fig. 3B). The serum CGRP concentrations in the RAMP1+/+ (1.36 ± 0.49 ng/ml) and the RAMP1−/− mice (1.16 ± 0.30 ng/ml) were comparable at 0 h, as shown in supporting information (SI) Fig. 7. These results indicate that the elevated blood pressure in the RAMP1−/− mice was due to the disruption in vasodilatory regulation caused by RAMP1 deficiency and not due to a decrease in the blood CGRP levels.
Fig. 3.
SBP and HR in RAMP1+/+ and RAMP1−/− mice. SBP and HR were measured by using a tail-cuff plethysmography apparatus in conscious mice for 18 weeks at intervals of 3 weeks. SBP and HR values in RAMP1+/+ (●) and RAMP1−/− (▴) mice were derived from an average of 10 measurements per animal for each time point.
Relaxation Responses to αCGRP, βCGRP, and AM in the Aortic Rings.
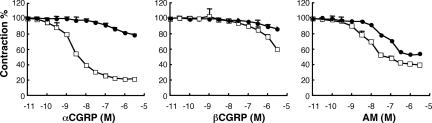
To clarify whether CLR/RAMP1 functions as the receptor for the two CGRP isoforms in the blood vessels, we compared their relaxation activities in the aortic rings. Fig. 4 shows that the cumulative addition of αCGRP to the aortic rings precontracted with phenylephrine exhibited a concentration-dependent relaxation, along with decreased sensitivity in the aortas that were obtained from the RAMP1−/− mice compared with those obtained from the RAMP1+/+ mice; the concentration that induces 50% of the maximum response to αCGRP (ED50) was significantly higher in the RAMP1−/− mice (2.8 ± 0. 2 × 10−7 M; P < 0.001) than in the RAMP1+/+ mice (3.1 ± 0.3 × 10−9 M). The maximal relaxation of the aortas caused by αCGRP was significantly decreased in the RAMP1−/− mice compared with the RAMP1+/+ mice (17.9 ± 4 vs. 78.9 ± 0.9%; P < 0.001). On the other hand, relatively high concentrations of βCGRP (>5 × 10−6 M) were required for a relaxation response in the aortas of the RAMP1+/+ mice, indicating that αCGRP exerted a considerably stronger relaxation effect than βCGRP on the blood vessels. However, decreased sensitivity to βCGRP was also observed in the aortas obtained from the RAMP1−/− mice compared with those obtained from the RAMP1+/+ mice. These results indicate that CLR/RAMP1 was composed of the receptors for both αCGRP and βCGRP. The cumulative addition of AM produced a dose-dependent relaxation in the aortic rings preconstricted with phenylephrine in the RAMP1+/+ mice. Interestingly, the ED50 values of AM significantly increased in the RAMP1−/− mice compared with the RAMP1+/+ mice (1.1 ± 0.1 × 10−7 vs. 9.8 ± 0.8 × 10−9 M; P < 0.001). These results further support the experimental results shown in Fig. 2 that CLR/RAMP1 functions as a receptor for not only CGRP but also AM in the blood vessels.
Fig. 4.
Relaxation induced by αCGRP, βCGRP, and AM in ring preparations of the thoracic aorta from RAMP1+/+ and RAMP1−/− mice. The contractile responses to the cumulative concentrations of αCGRP, βCGRP, and AM were compared in the thoracic aorta ring of the RAMP1+/+ (□) and the RAMP1−/− (●) mice. The results were expressed as the percentage of contraction evoked by 10 μM phenylephrine. At the end of each experiment, papaverine (0.1 mM) was added to detect the maximal relaxation. The values are means ± SD (n = 4) for each point.
High Concentrations of CGRP and Proinflammatory Cytokines in the Sera of LPS-Administered RAMP1−/− Mice.
Several lines of evidence indicate that CGRP may also play an important role in the regulation of inflammation and of the immune system. To compare the inflammatory responses, we administered i.p. a sublethal dose of LPS to the RAMP1+/+ mice and RAMP1−/− mice. We then quantified the serum CGRP and proinflammatory cytokine levels in a time-dependent manner. There was no significant difference in the serum CGRP concentrations in either the RAMP1+/+ or RAMP1−/− mice before the LPS administration, as described above. LPS induced an ≈4-fold elevation in the serum CGRP concentrations in the RAMP1+/+ mice (5.55 ± 8.97 ng/ml) 6 h after the administration (SI Fig. 7). Interestingly, the RAMP1−/− mice exhibited a rapid and significant increase in the serum CGRP concentrations, which peaked at 3 h after LPS administration (23.06 ± 27.97 ng/ml) and reached steady-state levels 12 h after the administration.
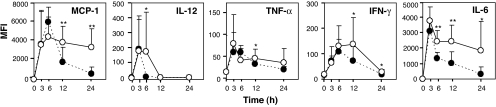
LPS is known to induce the production of cytokines, including TNF-α, IFN-γ, IL-12, IL-6, macrophage chemoattractant protein (MCP)-1, and IL-10. Therefore, the time-dependent serum cytokine levels were determined in the LPS-administered RAMP1+/+ and RAMP1−/− mice by using cytometric bead array (Fig. 5). In the RAMP1+/+ mice, it was observed that the elevated cytokine concentrations peaked at 3 to 6 h, rapidly declined thereafter, and reached steady-state levels 24 h after LPS administration. In the RAMP1−/− mice, the times corresponding to the peak cytokine concentrations were the same as those observed in the RAMP1+/+ mice, except for that of IFN-γ (peak at 12 h). However, a delayed decline in the proinflammatory cytokine (TNF-α, IFN-γ, IL-12, and IL-6) and chemokine (MCP-1) concentrations was detected in the RAMP1−/− mice, and the concentrations of MCP-1, IFN-γ, and IL-6 were significantly higher than those observed in the RAMP1+/+ mice even 24 h after LPS administration. On the other hand, no significant difference was observed in IL-10 levels between RAMP1+/+ and RAMP1−/− mice (data not shown). These results suggest that CGRP release might be regulated by an autocrine mechanism at nerve endings and that the released CGRP might regulate the production of proinflammatory cytokines by signal transduction through CLR/RAMP1 receptors.
Fig. 5.
Serum inflammatory cytokine levels in LPS-administered RAMP1+/+ and RAMP1−/− mice. The inflammatory cytokine levels in the sera of RAMP1+/+ (closed circles) and RAMP1−/− (open circles) mice displayed were measured by cytometric bead array. Mean fluorescence intensity (MFI) is expressed as mean ± SD (n = 9 mice per genotype). *, P < 0.05; **, P < 0.01.
Similar Inhibitory Effects of αCGRP and βCGRP on Cytokine Production in Bone Marrow-Derived DCs Through CLR/RAMP1 Receptors.
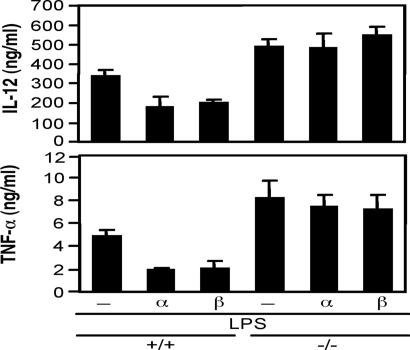
LPS activates toll-like receptor 4 and induces the production of proinflammatory cytokines such as TNF-α and IL-12 in DCs. We examined the effect of CGRP isoforms on TNF-α and IL-12 production in LPS-stimulated bone marrow-derived DCs (BMDCs). The DCs were differentiated from the bone marrow cells of the RAMP1+/+ and RAMP1−/− mice and were stimulated with LPS in the presence or absence of either of the CGRP isoforms. There was no difference in either the CD11c expression or the phagocytosis activity in the BMDCs of the RAMP1+/+ and RAMP1−/− mice. The BMDCs of both strains released TNF-α and IL-12 after LPS stimulation, their levels peaking 3–6 h after the stimulation (data not shown). Not only αCGRP but also βCGRP inhibited cytokine production in the BMDCs of the RAMP1+/+ mice (Fig. 6). In contrast, the inhibitory effect of the CGRP isoforms on the cytokine production was completely abolished in the BMDCs of the RAMP1−/− mice. The detailed inhibitory effects of the CGRP isoforms on TNF-α and IL-12 productions were examined in C57BL/6-derived BMDCs (SI Fig. 8). Both αCGRP and βCGRP exhibited similar inhibitory effects on IL-12 production in a dose-dependent manner. The inhibitory pattern of the CGRP isoforms on TNF-α secretion was similar; however, αCGRP appeared to have a slightly stronger activity than βCGRP. These results indicated that both the CGRP isoforms exerted a similar effect on TNF-α and IL-12 production through the CLR/RAMP1 receptors in LPS-stimulated BMDCs.
Fig. 6.
Effects of αCGRP and βCGRP on TNF-α and IL-12 production in LPS-stimulated BMDCs of RAMP1+/+ and RAMP1−/− mice. BMDCs differentiated from bone marrow cells in RAMP1+/+ and RAMP1−/− mice by the addition of mouse granulocyte macrophage-colony stimulating factor (20 ng/ml) for 10 days. The BMDCs were stimulated with LPS (0.1 μg/ml) in the presence or absence of αCGRP and βCGRP. The concentrations of TNF-α and IL-12 in the culture supernatants were determined by ELISA. The data represent means ± SD of triplicate experiments.
Discussion
The molecular biological analyses indicate that the CGRP receptor is composed of CLR, a common subunit of the receptors for CGRP and AM, and a unique receptor subunit RAMP1. The combination of CLR with RAMP2 functions as an AM receptor, and that of CLR and RAMP3 exhibits binding affinities similar to those exhibited by AM and CGRP (20); however, very little is known regarding its function. In the present study, we developed RAMP1−/− mice to demonstrate the physiological functions of CGRP involving the CLR/RAMP1 receptors. The RAMP1−/− mice exhibited significantly elevated SBP with normal HR. Moreover, the potent vasodilatory activity of CGRP almost completely disappeared in the RAMP1−/− mice both in vitro and in vivo. These results indicate that CLR/RAMP1 constituted the critical CGRP receptor to transduce the vasodilatory signal. On the other hand, the vasodilatory activity of AM mediated through the CLR/RAMP1 receptors cannot be ignored because the effects of AM on blood pressure and relaxation responses in the aortas were partially inhibited in the RAMP1−/− mice. Several lines of evidence also support the expression of vasodilator activity of AM via the CLR/RAMP1 receptors: the decrease in arterial pressure, vasodilation of the systemic vascular system, and AM-induced vasorelaxation of arteries are inhibited by the CGRP1 receptor antagonist CGRP8–37 (15); furthermore, HEK 293T cells cotransfected with CLR and RAMP1 exhibit an increase in the cAMP levels induced by AM, and this effect of AM is also antagonized by CGRP8–37 (21). These results suggest that the deficient signaling of AM through CLR/RAMP1 in blood vessels would be involved partly in the development of high blood pressure in RAMP1−/− mice.
Pharmacologically, CGRP is known to have the most potent vasodilatory activity. Therefore, the lack of CGRP function was assumed to lead to high blood pressure in vivo. Three groups have already reported the development of αCGRP-deficient mice, where the phenotypes of the deficient mice were diverse in the analyses of their cardiovascular systems. Lu et al. have generated the first strain of αCGRP-deficient mice and demonstrate that there are no differences between the WT and mutant mice with regard to their HR and blood pressure under basal or exercise-induced conditions (22). Furthermore, Gangula et al. have reported significantly higher SBP and mean arterial pressure levels in αCGRP/calcitonin-deficient mice compared with the WT mice (23). Because the calcitonin gene was also knocked out in the mice, it is not certain whether the phenotypes of the mutant mice were by the loss of CGRP, calcitonin, or both. Recently, a third strain of αCGRP-deficient mice has been developed by Oh-hashi et al. (24). The knockout mice have significantly higher mean arterial pressure as well as HR than the WT mice. Although the reasons for the observed differences in these knockout mice are not clear, the differences in their targeting strategies, genetic backgrounds, and/or breeding conditions are assumed to be responsible. In this study, we found that the RAMP1−/− mice exhibited high blood pressure with normal HR. The difference in HR between the RAMP1−/− mice and the αCGRP-deficient mice produced by Oh-hashi et al. may be related to CGRP signal transduction through types of receptors other than CLR/RAMP1. In the experiments on the relaxation response of the aortas of the RAMP1−/− mice, we discovered that the activities of both αCGRP and βCGRP did not completely disappear at higher concentrations. Therefore, a heterodimer of CLR and RAMP3 might be involved in the regulation of HR as a receptor for CGRP.
Two isoforms of CGRP are synthesized in the cell bodies of primary afferent neurons, although their physiological functions do not appear to be similar. It was reported that the cardiovascular effects of CGRP are mainly mediated by αCGRP in rats (25). In human beings, CGRP I significantly stimulates blood flow through the carotid artery and the elevation in HR compared with CGRP II (26). Because, in our study, the effect of αCGRP on the relaxation response of the aortas was observed to be ≈3 times more potent than that of βCGRP, the elevated blood pressure in the RAMP1−/− mice would have resulted mainly from the lack of αCGRP signaling through the CLR/RAMP1 receptors. On the other hand, both αCGRP and βCGRP suppressed TNF-α and IL-12 production in LPS-stimulated BMDCs in a similar dose-dependent manner. The inhibitory effects of both the CGRP isoforms on cytokine production almost completely diminished in the LPS-stimulated BMDCs obtained from the RAMP1−/− mice, indicating that RAMP1 is composed of a receptor subunit specific to both CGRP isoforms, at least in the immune system regulated by the DCs. These results suggest that both the CGRP isoforms are capable of binding CLR/RAMP1; however, different intracellular signaling molecule(s) might be involved in the regulation of immune system and cardiovascular system downstream of the CLR/RAMP1 receptors. Recently, we found that BMDCs stimulated with LPS or antigens, secreted only βCGRP (data not shown). Therefore, CGRP isoforms would exert, together or independently, biological effects in the nervous, cardiovascular, and immune systems (9).
In addition to the cardiovascular effects of CGRP, its functions in inflammation and the immune system also would play an important role in pathophysiology. CGRP appears to be involved in the development of sepsis (27), airway diseases (28), and colitis (29). The invasion of pathogens and antigens stimulates the sensory nerves directly and/or indirectly to secrete CGRP from the nerve endings. CGRP-containing nerve fibers are intimately associated with antigen-presenting cells such as Langerhans cells and macrophages in the epidermis (8). CGRP modulates antigen presentation (30), phagocytosis (7), and the production of cytokines induced by the LPS (31) of these cells. In the RAMP1−/− mice, high serum levels of proinflammatory cytokines would result in defective signal transduction of CGRP through the CLR/RAMP1 receptors in not only macrophages but also DCs. We have clarified that CGRP exerts its antiinflammatory effect by inhibiting the production of TNF-α and IL-12 in BMDCs stimulated with LPS. DCs play a key role in regulating both innate and acquired immunity toward foreign antigens. Our previous report indicated that CGRP also regulates cytokine production in helper T cells stimulated with CD3/CD28 antibodies (9). These findings indicate that CLR/RAMP1 receptor signaling modulates the inflammatory responses and the innate and acquired immunity of CGRP and that a disorder in signal transduction might lead to the diseases mentioned above.
Taken together, we have clarified the development of hypertension and the dysfunctional regulation of proinflammatory cytokines in RAMP1−/− mice. RAMP1 is an essential subunit of the CGRP receptor that regulates the vasodilatory activity of αCGRP in blood vessels and the antiinflammatory activity of both αCGRP and βCGRP in DCs. Agonists for CLR/RAMP1 receptors might be useful as therapeutic medicines for cardiovascular diseases or intractable inflammatory diseases.
Materials and Methods
Production of RAMP1-Deficient Mice.
To generate the Ramp1 targeting construct, we cloned an ≈9,000-bp BamHI fragment from the mouse genomic Ramp1 locus, which contains Ramp1 exon 2, by using the bacterial artificial chromosome clone (BAC; Kurabo, Osaka, Japan). First, loxP was inserted into an NsiI site 200 bp upstream of Ramp1 exon 2, and the loxP–Neor–loxP cassette was inserted into an MfeI site 2,000 bp downstream of exon 2. PGK-TK (mouse phosphoglycerate kinase–thymidine kinase) from pPNT was inserted into a BamHI site 5,700 bp upstream of exon 2. D3 ES cells were transfected with 20 μg of NotI-linearized targeting vector by using a Gene Pulser (Bio-Rad, Hercules, CA). Positive selection by using 150 μg/ml of G418 and negative selection by using 2 μM ganciclovir was conducted on the first and second days after the electroporation, respectively. RAMP1-floxed ES cell clones were microinjected into C57BL/6 blastocysts to generate chimeric mice. The chimeric mice were mated with WT C57BL/6 mice. The F1 mice in which the floxed RAMP1 gene was successfully transmitted into the germ line were intercrossed to generate RAMP1–flox/flox mice. The RAMP1–flox/flox mice were mated with CAG–Cre (pCAGGS–Cre recombinase) transgenic mice (32). To generate RAMP1+/− mice, the offspring carrying the CAG–Cre and floxed RAMP1 genes were mated with the WT C57BL/6 mice. The RAMP1-deficient mice were generated by the intercrossing of the RAMP1+/− mice. For immunological analyses, the RAMP1+/− mice backcrossed with BALB/c mice for eight generations were used to generate RAMP1−/− mice. All of the experiments were performed with the consent of the Animal Care and Use Committee of Osaka University.
Arterial Blood Pressure Measurement.
The mice were anesthetized by using sodium pentobarbital (45 mg/kg, i.p.). An incision was made in the neck to expose the left carotid artery, which was then punctured by using a 27-gauge needle. A catheter, a blunt 26-gauge needle connected to a polyethylene tube, was inserted into the carotid artery toward the heart. After confirming the inflow of blood into the catheter, a heparin–Na solution was injected into the polyethylene tube by using a 1-ml syringe to prevent the coagulation of blood. The catheter was connected to a pressure transducer (model DX-100; Nihon Kohden, Tokyo, Japan). The arterial blood pressure was recorded on a polygraph (model RM-600G; Nihon Kohden). Each drug was administered into the tail vein of the mice.
Measurement of SBP and HR.
SBP and HR were measured by using a tail-cuff plethysmography apparatus (MK-2000; Muromachi Kikai, Tokyo, Japan) in conscious mice. The SBP and HR values were derived from an average of 10 measurements per animal at each time point.
Measurement of Relaxation Response to αCGRP, βCGRP, and AM in Aortic Rings.
The mice were killed by exsanguination from the carotid artery under ether anesthesia. The thoracic aorta was excised and immediately placed in the Krebs–Henseleit solution (118.4 mM NaC1/4.7 mM KC1/2.5 mM CaCl2/1.2 mM KH2PO4/1.2 mM MgSO4, 25.0 mM NaHCO3, and 11.1 mM glucose). The aorta was cleaned of adherent tissue and cut into 3-mm rings. Each ring was vertically fixed under a resting tension of 0.7 g in a 5-ml organ bath filled with the abovementioned solution (37°C, pH 7.4). The isometric tension change was measured by using a force-displacement transducer (Isometric Transducer UFER; Medical Kishimoto, Kyoto, Japan) coupled to a dual-channel chart recorder (Mac Lab; AD Instruments, Tokyo, Japan). The integrity of the endothelium was assessed by acetylcholine-induced relaxation (30 μM) in aortas precontracted with phenylephrine (10 μM). After reequilibration for 30 min, αCGRP, βCGRP, and AM were cumulatively added to the organ bath at concentrations ranging between 10 pM and 1 μM. At the end of each experiment, papaverine (0.1 mM) was added to detect the maximal relaxation.
Determination of CGRP and Proinflammatory Cytokine Levels in the Serum of LPS-Administered Mice.
LPS (Escherichia coli Re595; Sigma–Aldrich Japan, Tokyo, Japan) was administered i.p. in RAMP1+/+ and RAMP1−/− mice. Blood was collected from the tail vein at the indicated time points (Fig. 5) and was allowed to clot for 1 h. The serum CGRP levels were determined by CGRP ELISA (Phoenix Pharmaceuticals, Burlingame, CA). The serum levels of proinflammatory cytokines [IL-12p70 (IL-12), TNF-α, INF-γ, MCP-1, IL-10, and IL-6] were determined by using a mouse inflammation cytokine cytometric bead array kit (BD Biosciences, Franklin Lakes, NJ) according to the instructions of the manufacturer. The minimum sensitivity of the assay is 5 pg/ml (IL-6), 17.5 pg/ml (IL-10), 52.7 pg/ml (MCP-1), 2.5 pg/ml (IFN-γ), 7.3 pg/ml (TNF), and 10.7 pg/ml (IL-12p70).
BMDC Generation.
BMDCs were generated in vitro (33). Briefly, the bone marrow cells were cultured in RPMI medium 1640 containing 10% FCS and mouse granulocyte macrophage-colony stimulating factor (20 ng/ml) for 10 days. Nonadherent and loosely adherent cells were harvested and used as BMDCs (>85% CD11c+). The BMDCs were cultured in 48-well culture plates at 2.5 × 105 cells/500 μl and stimulated with LPS (0.1 μg/ml) in the presence or absence of αCGRP and βCGRP.
Cytokine Measurement by ELISA.
Cell-free supernatants were harvested 6 and 48 h after stimulation, and the TNF-α and IL-12 concentrations were determined by using ELISA. TNF-α was measured by using a mouse TNF-α ELISA kit (Biolegend, San Diego, CA) according to the instructions of the manufacturer. An in-house ELISA was used to quantify IL-12, as described previously (9).
Statistical Analysis.
The statistical significance of differences was determined by Student's t test for unpaired data.
Supplementary Material
Acknowledgments
We thank Dr. Hiroharu Funaya (Hyogo College of Medicine) for helpful comments, Dr. Nobutaka Suzuki (RIKEN Research Center), Dr. Shinobu Suzuki (Nihon Schering) for instructions on the culturing of BMDCs, and Yumiko Maruyama and Kazuko Kishida for technical assistance. This work was supported, in part, by the Long-Range Research Initiative, the Japan Chemical Industry Association, PCA InterMed, Inc., and Fuso Pharmaceutical Industries, Ltd.
Abbreviations
- AM
adrenomedullin
- BMDC
bone marrow-derived dendritic cell
- CLR
calcitonin receptor-like receptor
- CGRP
calcitonin gene-related peptide
- DC
dendritic cell
- HR
heart rate
- MCP
macrophage chemoattractant protein
- RAMP
receptor activity-modifying protein
- SBP
systolic blood pressure.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705974104/DC1.
References
- 1.Wimalawansa SJ. Endocr Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 2.Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S, et al. Neuroscience. 1988;25:195–205. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 3.Xing L, Guo J, Wang X. J Immunol. 2000;165:4359–4366. doi: 10.4049/jimmunol.165.8.4359. [DOI] [PubMed] [Google Scholar]
- 4.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 5.Bell D, McDermott BJ. Pharmacol Rev. 1996;48:253–288. [PubMed] [Google Scholar]
- 6.Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Nat Neurosci. 2001;4:357–358. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- 7.Ichinose M, Sawada M. Peptides. 1996;17:1405–1414. doi: 10.1016/s0196-9781(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 8.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 9.Tokoyoda K, Tsujikawa K, Matsushita H, Ono Y, Hayashi T, Harada Y, Abe R, Kubo M, Yamamoto H. Int Immunol. 2004;16:643–653. doi: 10.1093/intimm/dxh072. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura N, Tamura H, Obana S, Wenner M, Ishikawa T, Nakata A, Yamamoto H. Neuroimmunomodulation. 1998;5:9–15. doi: 10.1159/000026321. [DOI] [PubMed] [Google Scholar]
- 11.Dennis T, Fournier A, Cadieux A, Pomerleau F, Jolicoeur FB, St Pierre S, Quirion R. J Pharmacol Exp Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- 12.Dennis T, Fournier A, St Pierre S, Quirion R. J Pharmacol Exp Ther. 1989;251:718–725. [PubMed] [Google Scholar]
- 13.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 14.Miyauchi K, Tadotsu N, Hayashi T, Ono Y, Tokoyoda K, Tsujikawa K, Yamamoto H. Neuropeptides. 2002;36:22–33. doi: 10.1054/npep.2002.0871. [DOI] [PubMed] [Google Scholar]
- 15.Hinson JP, Kapas S, Smith DM. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 16.Steiner S, Muff R, Gujer R, Fischer JA, Born W. Biochemistry. 2002;41:11398–11404. doi: 10.1021/bi020279r. [DOI] [PubMed] [Google Scholar]
- 17.Sexton PM, Albiston A, Morfis M, Tilakaratne N. Cell Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 18.Aiyar N, Rand K, Elshourbagy NA, Zeng Z, Adamou JE, Bergsma DJ, Li Y. J Biol Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- 19.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 20.Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. Br J Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagoshi Y, Kuwasako K, Ito K, Uemura T, Kato J, Kitamura K, Eto T. Eur J Pharmacol. 2002;450:237–243. doi: 10.1016/s0014-2999(02)02184-2. [DOI] [PubMed] [Google Scholar]
- 22.Lu JT, Son YJ, Lee J, Jetton TL, Shiota M, Moscoso L, Niswender KD, Loewy AD, Magnuson MA, Sanes JR, et al. Mol Cell Neurosci. 1999;14:99–120. doi: 10.1006/mcne.1999.0767. [DOI] [PubMed] [Google Scholar]
- 23.Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, Gagel RF, Yallampalli C. Hypertension. 2000;35:470–475. doi: 10.1161/01.hyp.35.1.470. [DOI] [PubMed] [Google Scholar]
- 24.Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, et al. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 25.Deng PY, Ye F, Zhu HQ, Cai WJ, Deng HW, Li YJ. Regul Pept. 2003;114:175–182. doi: 10.1016/s0167-0115(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 26.Beglinger C, Born W, Munch R, Kurtz A, Gutzwiller JP, Jager K, Fischer JA. Peptides. 1991;12:1347–1351. doi: 10.1016/0196-9781(91)90218-e. [DOI] [PubMed] [Google Scholar]
- 27.Arnalich F, Sanchez JF, Martinez M, Jimenez M, Lopez J, Vazquez JJ, Hernanz A. Life Sci. 1995;56:75–81. doi: 10.1016/0024-3205(94)00416-p. [DOI] [PubMed] [Google Scholar]
- 28.Dakhama A, Larsen GL, Gelfand EW. Curr Opin Pharmacol. 2004;4:215–220. doi: 10.1016/j.coph.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Eysselein VE, Reinshagen M, Patel A, Davis W, Nast C, Sternini C. Ann NY Acad Sci. 1992;657:319–327. doi: 10.1111/j.1749-6632.1992.tb22779.x. [DOI] [PubMed] [Google Scholar]
- 30.Asahina A, Moro O, Hosoi J, Lerner EA, Xu S, Takashima A, Granstein RD. Proc Natl Acad Sci USA. 1995;92:8323–8327. doi: 10.1073/pnas.92.18.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monneret G, Pachot A, Laroche B, Picollet J, Bienvenu J. Cytokine. 2000;12:762–764. doi: 10.1006/cyto.1999.0607. [DOI] [PubMed] [Google Scholar]
- 32.Sakai K, Miyazaki J. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 33.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.