Humans, like all animals, depend on sensory systems to perceive different aspects of the physical world. One of the most remarkable achievements of evolution has been the development of sensory cells that are specialized to detect unique aspects of this world such as a single photon or a single molecular odorant. Similarly, our ability to perceive sound is mediated through the vibration of exquisitely sensitive stereociliary bundles located on the luminal surfaces of mechanosensory hair cells. A deflection of a little as 1 nM at the tip of the stereociliary bundle is sufficient to elicit a change in the resting potential and synaptic activity of the cell (1). Unraveling the molecular mechanisms that mediate the perception of physical stimuli has been one of the most exciting and rewarding endeavors in modern biology. The characterization of the phototransduction cascade has not only provided insights into the function of the visual system but also contributed profoundly to our understanding of G protein-coupled receptor signaling (2). Surely similar insights regarding mechanotransduction will be obtained once the molecular pathways underlying this sensory modality have been revealed. However, the limited number of mechanosensory hair cells present in any single organism has impeded our ability to dissect the molecular basis of mechanotransduction. Although ≈30 million photoreceptors can be obtained from a single rodent retina, both cochleae from the same animal contain <20,000 mechanosensory hair cells (3, 4). The relative scarcity of these cells emphasizes not only their exceptional level of efficiency but also another daunting aspect of inner ear biology: In mammals, mechanosensory hair cells are generated only during embryogenesis. Over time, and with marked influences from environment and genetic makeup, mechanosensory hair cells are inexorably lost, eventually resulting in loss of hearing acuity or deafness (5). As is the case for mechanotransduction, the limited number of these cells has hampered the pace of discovery in our understanding of the factors that both promote and inhibit the formation of hair cells. However, an article by Hu and Corwin (6) in this issue of PNAS describes a new method for the potential generation of large numbers of bona fide hair cells in vitro.
One of the most obvious and potentially most promising approaches to circumvent the limited number of naturally occurring hair cells is to develop cell lines that can be used to generate large numbers of bona fide hair cells. Similar approaches with other cell types, such as tumor cells or pancreatic islet cells, have yielded significant results. Several research teams have attempted to generate cell lines with the potential to develop as hair cells from a number of sources including embryonic and other types of well characterized stem cells, as well as stem cells or progenitor cells isolated from inner ear tissue (7–15). The results of several of these studies have demonstrated that with appropriate in vitro stimulation, multipotential cells from a number of different sources can be induced to express many of the proteins that are known to be highly expressed in hair cells. However, none of these proteins is truly unique to hair cells in the way that opsins are unique to photoreceptors, making it impossible to definitely confirm that these cells are truly hair cells. Moreover, the expression of a collection of genes or proteins is not the best way to definitively establish cellular phenotype, even if that collection includes factors that are unique to a particular cell type. Rather, a rigorous demonstration of phenotype would involve the cell in question exhibiting both an appropriate morphology and the ability to respond appropriately to a specific physical stimulus. In the case of hair cells, they should generate a stereociliary bundle and be capable of mechanotransduction. It is important to note that in several instances, actin-rich specializations have been observed within in vitro-derived hair cells, but none could be considered to be true stereociliary bundles (9). In some cases, the ability of these cells to develop as hair cells was confirmed by transplantation into an embryonic inner ear, suggesting that the conditions in vitro were not sufficient to induce complete hair cell formation (8, 14).
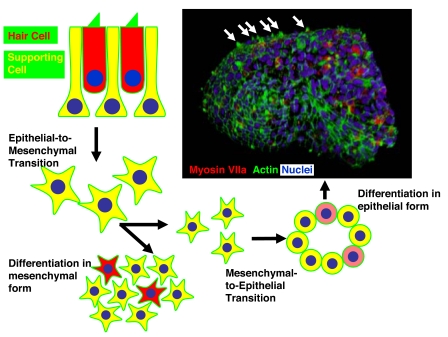
The study by Hu and Corwin (6) takes a large stride toward defining at least one key aspect of the cellular environment that is required for hair cell differentiation; they demonstrate that a forced mesenchymal-to-epithelial transition (MET) leads to the formation of cells with several unique aspects of hair cell morphology. Following a fairly standard isolation and in vitro expansion of supporting cells from the chick utricle that included an epithelial-to-mesenchymal transition, the authors forced the cells to revert to an epithelial phenotype by using either gentle agitation or a hanging-drop culture system to prevent adhesion to the substrate. The result of this forced transition was the formation of spheres that included a polarized and apparently pseudostratified epithelium (Fig. 1). Cells within these spheres expressed markers that were consistent with both hair cells and supporting cells. More importantly, many of the putative hair cells developed unique aspects of hair cell morphology, including structures that appeared to be stereociliary bundles, but, surprisingly, these stereociliary bundles were
In mammals, mechanosensory hair cells are generated only during embryogenesis.
oriented outward into the culture medium rather than inward to the lumen. Scanning electron microscopic images provide convincing evidence that these structures contain both stereocilia and kinocilia, and in some cases, that the stereocilia appear to be arranged in a staircase pattern. Although Hu and Corwin did not confirm the ability of these cells to respond to mechanical stimulation, putative hair cells accumulated FM1–43, a styryl dye that permeates hair cells through the mechanotransduction channels (15–17). On the basis of these results, and with the caveat that mechanotransduction and cellular excitability must still be demonstrated, it appears likely that the cells within these spheres develop as true hair cells. Although the total number of hair cells per sphere was modest, never exceeding 15%, the expansion potential of these cells when in their proliferative, mesenchymal form was robust with a consistent doubling time of just less than 5 days. Therefore, it is possible to envision the generation and isolation of significant numbers of hair cells from a relatively modest starting population, especially if techniques that will increase the percentage of hair cells per sphere are identified. Finally, it is important to consider that it is not known whether a similar approach will work with mammalian cells. As the authors suggest, there is no reason to think that a forced MET will be less effective at inducing formation of hair cell-containing spheres from mammalian tissue, but considering the relatively limited innate proliferative potential of mammalian supporting cells, expansion of these cells while in a mesenchymal form may prove more challenging (18–23).
Fig. 1.
Summary of the different transitions used to generate hair cells in vitro. First, epithelia containing hair cells (red) and supporting cells (yellow) are dissociated. Hair cells subsequently die, and supporting cells undergo an epithelial-to-mesenchymal transition. Once in a mesenchymal form, supporting cells can increase in number through mitotic proliferation. Under conditions that induce differentiation of these cells while still in a mesenchymal form, some of the cells will begin to express hair cell-specific markers (red), but the cells will not develop a definitive hair cell morphology. In contrast, Hu and Corwin (6) demonstrate that if these cells are forced to undergo a mesenchymal-to-epithelial transition (MET), spheres that include both hair cells (expression of a hair cell marker, myosin VIIa, in red) and supporting cells form. Moreover, the hair cells develop actin-containing stereociliary bundles (labeled in green with phalloidin), resulting in spheres that appear studded (white arrows in micrograph). Micrograph courtesy of Z. Hu and J. Corwin.
In summary, Hu and Corwin (6) present exciting new data suggesting that a key step in the de novo generation of hair cells in vitro is a forced MET. Although the authors have not yet definitively demonstrated the generation of excitable, mechanotransducing hair cells within their spheres, they have produced cells with a morphology and molecular profile that are strikingly consistent with those of a hair cell. Assuming that these cells will be conclusively shown to be mechanosensory hair cells, this new technique has the potential to significantly accelerate the study of hair cell biology.
Footnotes
The author declares no conflict of interest.
See companion article on page 16675.
References
- 1.Fettiplace R, Hackney CM. Nat Rev Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 2.Arshavsky VY, Lamb TD, Pugh EN., Jr Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 3.Kaltenbach JA, Falzarano PR. J Comp Neurol. 1994;340:87–97. doi: 10.1002/cne.903400107. [DOI] [PubMed] [Google Scholar]
- 4.Nadol JB., Jr Hear Res. 1988;34:253–266. doi: 10.1016/0378-5955(88)90006-8. [DOI] [PubMed] [Google Scholar]
- 5.Matsui JI, Parker MA, Ryals BM, Cotanche DA. Drug Discov Today. 2005;10:1307–1312. doi: 10.1016/S1359-6446(05)03577-4. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z, Corwin JT. Proc Natl Acad Sci USA. 2007;104:16675–16680. doi: 10.1073/pnas.0704576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC. Genome Res. 2002;12:1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Roblin G, Liu H, Heller S. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Liu H, Heller S. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 10.Doetzlhofer A, White PM, Johnson JE, Segil N, Groves AK. Dev Biol. 2004;272:432–447. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Liu JJ, Shin JH, Hyrc KL, Liu S, Lei D, Holley MC, Bao J. Otol Neurotol. 2006;27:414–421. doi: 10.1097/00129492-200604000-00020. [DOI] [PubMed] [Google Scholar]
- 12.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 13.Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, Cotanche DA. Hear Res. 2007;232:29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon SJ, Oshima K, Heller S, Edge AS. Mol Cell Neurosci. 2007;34:59–68. doi: 10.1016/j.mcn.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 19.Kuntz AL, Oesterle EC. J Comp Neurol. 1998;399:413–423. [PubMed] [Google Scholar]
- 20.Hume CR, Kirkegaard M, Oesterle EC. J Assoc Res Otolaryngol. 2003;4:422–443. doi: 10.1007/s10162-002-3008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montcouquiol M, Corwin JT. J Neurosci. 2001;21:974–982. doi: 10.1523/JNEUROSCI.21-03-00974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu R, Montcouquiol M, Marchionni M, Corwin JT. Eur J Neurosci. 2007;25:1363–1372. doi: 10.1111/j.1460-9568.2007.05414.x. [DOI] [PubMed] [Google Scholar]
- 23.Meyers JR, Corwin JT. J Neurosci. 2007;27:4313–4325. doi: 10.1523/JNEUROSCI.5023-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]