Abstract
Body water homeostasis depends critically on the hormonally regulated trafficking of aquaporin-2 (AQP2) water channels in renal collecting duct epithelial cells. Several types of posttranslational modifications are clearly involved in controlling the distribution of AQP2 between intracellular vesicles and the apical plasma membrane. Little is known, however, about the protein interactions that govern the trafficking of AQP2 between these organelles. MAL is a detergent-resistant membrane-associated protein implicated in apical sorting events. We wondered, therefore, whether MAL plays a role in the regulated trafficking of AQP2 between intracellular vesicles and the apical surface. We find that AQP2 and MAL are coexpressed in epithelial cells of the kidney collecting duct. These two proteins interact, both in the native kidney and when expressed by transfection in cultured cells. The S256-phosphorylated form of AQP2 appears to interact more extensively with MAL than does the water channel protein not phosphorylated at this serine. We find that MAL is not involved in detergent-resistant membrane association or apical delivery of AQP2 in LLC-PK1 renal epithelial cells. Instead, MAL increases the S256 phosphorylation and apical surface expression of AQP2. Furthermore, internalization experiments show that MAL induces surface expression of AQP2 by attenuating its internalization. Thus, the involvement of MAL in the cell surface retention of apical membrane proteins could play an important role in regulated absorption and secretion in transporting epithelia.
Keywords: epithelia, sorting, tetraspanin, trafficking
Aquaporin (AQP) water channels constitute a pathway for water transport driven by osmotic gradients. This water transport is largely regulated through the tissue distribution of specific AQPs and, in some cases, through control of their cell surface expression. In the epithelial cells of the renal collecting duct, water reabsorption is tightly regulated by the redistribution of AQP2 between intracellular vesicles and the apical cell surface (reviewed in ref. 1). This redistribution is controlled by the antidiuretic hormone arginine vasopressin, which binds to the vasopressin type-2 receptor and activates adenylyl cyclase. The subsequent downstream signaling cascade results in the phosphorylation of AQP2. This phosphorylation induces a shift in the distribution of AQP2 from intracellular vesicles to the apical plasma membrane, rendering this membrane domain water-permeable. Driven by the transtubular osmotic gradient, urinary water then enters the cells via AQP2 and exits the cells to the interstitium via AQP3 and AQP4, which are constitutively expressed in the basolateral plasma membrane. Removal of arginine vasopressin induces short-chain ubiquitination (2) and the internalization of AQP2 via clathrin-coated vesicles (3), resulting in the restoration of the water-impermeable state of the apical plasma membrane. The strictly apical shuttling of AQP2 and the tight regulation of its subcellular localization suggest an interaction with regulatory proteins. Inhibition of A-kinase anchoring protein and v-SNAREs abolishes the cAMP-induced redistribution of AQP2 to the apical plasma membrane, whereas actin depolymerization by the small GTPase Rho induces this redistribution (4–6), suggesting that these proteins play a role in modulating the subcellular localization of AQP2. However, these proteins are involved in a number of essential cellular processes, and it is not clear whether they play a specific role in the redistribution of AQP2.
Myelin and lymphocyte-associated protein (MAL), also known as vesicle integral protein of 17 kDa (VIP17), is present in Schwann cells, in oligodendrocytes, and in epithelial cells of the kidney, stomach, and large intestine (7). It spans the membrane four times and is structurally similar to members of the tetraspan family. In epithelial cells, MAL is restricted in its distribution to the apical plasma membrane (8). In every cell in which it is expressed, MAL is associated with glycosphingolipids and is thought to be involved in the organization, transport, and maintenance of glycosphingolipid-enriched membranes, which are resistant to detergent extraction. Because these glycosphingolipid-enriched membranes are thought to be part of the apical transport machinery (9), MAL might be involved in the organization of apical cargo into these membrane domains to form apical transport vesicles or apical microdomains (10). Indeed, in the epithelial Madin–Darby canine kidney (MDCK) cells, MAL associates with and plays a role in the apical transport of influenza virus HA (11).
In the kidney, MAL expression is observed in both the cortex and the medulla and is most abundant in the collecting duct (8), the tubule that also expresses AQP2. We report that MAL interacts with AQP2 in the kidney but is not involved in detergent-resistant membrane (DRM) association or apical delivery of AQP2 in renal epithelial LLC-PK1 cells. Instead, MAL increases the steady-state phosphorylation of AQP2 and enhances its apical surface expression by decreasing its internalization.
Results
MAL and AQP2 Colocalize and Physically Interact in the Renal Collecting Duct.
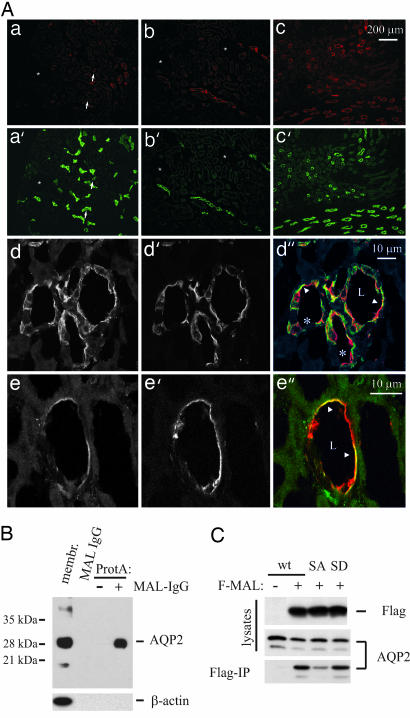
A role for MAL as a potential participant in the sorting of AQP2 in the kidney is plausible only if both proteins are present in the same cells. In the kidney, AQP2 is exclusively expressed in the collecting duct. The epithelium of the collecting duct consists of principal and intercalated cells, and AQP2 is expressed only in the principal cells. Frank et al. (8) found that MAL expression is observed in the renal collecting duct, but the cell type was not specified. Immunofluorescence studies performed on rat kidney show the presence of MAL in both the cortex and medulla (Fig. 1A). Costaining with Calbindin-28K, a marker for the connecting tubule, reveals that there exists a partial overlap, suggesting expression of MAL in the connecting tubule. Moreover, double staining of MAL and AQP2 reveals an almost complete overlap in signal, confirming the expression of MAL in the renal collecting duct. Immunofluorescence studies on kidneys of rats water-deprived for 16 h revealed that MAL is also present only in the principal cells of the collecting duct, although not all AQP2-positive cells manifest equal intensity of MAL staining (Fig. 1A). As a consequence of water deprivation, which stimulates vasopressin secretion, AQP2 was detected mainly in the apical plasma membrane, although intracellular staining was also detected. MAL staining was also observed at the apical plasma membrane, in intracellular structures, and, occasionally, in the basolateral plasma membrane. Interestingly, AQP2 and MAL colocalized in the apical plasma membrane of a subset of the collecting duct cells (Fig. 1A, arrowheads).
Fig. 1.
MAL interacts preferentially with phosphorylated AQP2. (A) Sections from paraformaldehyde lysine phosphate-perfused rat kidneys were stained for MAL and AQP2 or MAL and Calbindin-28K, and images were collected with a fluorescence microscope (a–c). MAL immunostaining was observed in the cortex (a and b) and medulla (c) and codistributed with Calbindin-28K (a′) and AQP2 (b′ and c′). The stars indicate glomeruli, and arrows point to tubules that exhibit expression of both MAL and Calbindin-28K. Sections of paraformaldehyde lysine phosphate-perfused kidneys from 16-h water-deprived rats were stained for MAL (d and e) and AQP2 (d′ and e′), and images were collected by confocal laser scanning microscope and merged (d″ and e″). The stars indicate intercalated cells, and arrowheads indicate cells with colocalization of AQP2 and MAL at the apical/luminal (L) surface. Scale bars are shown. The images are representatives from three independent experiments. (B) Rat kidney medulla membranes were solubilized in CHAPS and incubated with protein A-conjugated beads (ProtA) with (+) or without (−) MAL antibodies (MAL-IgG). Precipitates, 1% of the original solubilized membranes (membr.), and anti-MAL IgG were immunoblotted for AQP2 or β-actin. Marker bands are indicated on the left by their molecular masses in kilodaltons (n = 2). (C) COS-7 cells were transfected with a construct encoding WT-AQP2 alone (−) or transfected with (+) a construct encoding Flag-tagged MAL (F-MAL) in combination with WT-AQP2, AQP2-S256A (SA), or AQP2-S256D (SD). Cells were lysed in CHAPS and subjected to immunoprecipitation with Flag antibodies. Immunoprecipitates (Flag-IP) and 2% of the original lysates were subjected to immunoblotting for AQP2 or Flag.
To determine whether MAL and AQP2 interact in vivo, we solubilized membranes of kidney inner medullae from rats water-deprived for 16 h. Immunoprecipitation with MAL antibodies, and subsequent immunoblotting for AQP2, revealed that AQP2 indeed coprecipitated with MAL (Fig. 1B). To prove the specificity of the coprecipitation, and to exclude the possibility that the signal originates from cross-reactivity of the secondary reagent with IgG light chain (whose molecular weight is similar to that of AQP2), we ran control lanes loaded only with MAL antibodies, and we also carried out the precipitation without MAL antibodies. The specificity of the immunoprecipitation was also supported by the absence of any coprecipitated β-actin. We conclude, therefore, that AQP2 and MAL colocalize and interact in renal collecting duct principal cells.
MAL Interacts Preferentially with Phosphorylated AQP2.
Translocation of AQP2 from intracellular vesicles to the plasma membrane requires phosphorylation of AQP2 at S256. To analyze whether this phosphorylation might play a role in the association of MAL with AQP2 we exploited two AQP2 mutants. AQP2-S256A and AQP2-S256D mimic nonphosphorylated and phosphorylated AQP2, respectively (12). For these and subsequent studies MAL was N-terminally tagged with a Flag epitope, which was previously shown not to disturb its sorting or function (10, 11). Flag-MAL was coexpressed with WT-AQP2, AQP2-S256A, or AQP2-S256D in COS-7 cells. Flag immunoprecipitates revealed that WT-AQP2 coprecipitated with Flag-MAL and did not precipitate with Flag antibodies in the absence of Flag-MAL (Fig. 1C). AQP2-S256D was coimmunoprecipitated with Flag-MAL to a similar extent as WT-AQP2, whereas the amount of AQP2-S256A that coimmunoprecipitated with Flag-MAL was 10 times lower than that of WT-AQP2 (P < 0.05; the ratio of MAL-associated AQP2 to total AQP2 is 1.99 ± 0.25, 1.98 ± 0.13, and 0.20 ± 0.01 for WT-AQP2, AQP2-S256D, and AQP2-S256A, respectively; n = 3), as determined by densitometric quantitation of the immunoblot. Thus, these data suggest that the association of AQP2 with MAL coincides with the availability of AQP2 to become phosphorylated.
MAL Is Not Involved in the Apical Surface Delivery or DRM Association of AQP2.
To determine the role of MAL in the trafficking of AQP2, we searched for renal epithelial cells that do not express endogenous MAL. It has been shown that the mouse renal proximal tubule does not express MAL (8). The porcine epithelial LLC-PK1 cell line originates from the renal proximal tubule and, therefore, may also similarly lack MAL expression. Indeed, we could not detect MAL in LLC-PK1 cells by means of Northern blotting (data not shown) and, therefore, used these cells for further studies.
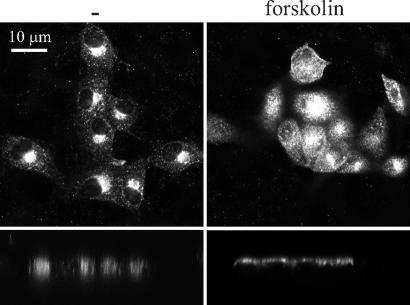
To analyze the distribution of AQP2 in the LLC-PK1 cells, these cells were transfected with a construct encoding AQP2. Fluorescence microscopy revealed that AQP2 is present in an intracellular compartment and translocates to the apical plasma membrane upon stimulation with forskolin (Fig. 2). Forskolin mimics the in vivo effect of arginine vasopressin through activation of adenylyl cyclase (13). Therefore, the localization and regulation of AQP2 in LLC-PK1 cells recapitulates the in vivo situation. The presence of AQP2 in the apical plasma membrane of LLC-PK1 cells after forskolin treatment suggests that MAL, whose expression is not detectable in these cells, is not likely to play an obligate role in the apical surface delivery of AQP2. Flag-MAL was also appropriately distributed to the apical plasma membrane of LLC-PK1 cells and retained its capacity to interact with AQP2, but not with the basolateral AQP4 water channel protein [supporting information (SI) Figs. 6 and 7]. These data validate the LLC-PK1 cell line as a model system with which to study the role of MAL in the trafficking events of AQP2.
Fig. 2.
AQP2 is redistributed to the apical plasma membrane of LLC-PK1 cells. Stable LLC-PK1 cell lines expressing AQP2 were grown to confluence and either not treated (Left) or treated with forskolin (Right). Subsequently, immunofluorescence using AQP2 antibodies was performed, and images were collected with a confocal laser-scanning microscope. For each protein, x–y sections (Upper) and x–z sections (Lower) are shown. (Scale bar: 10 μm.) The images are representative of those obtained with three independent cell lines.
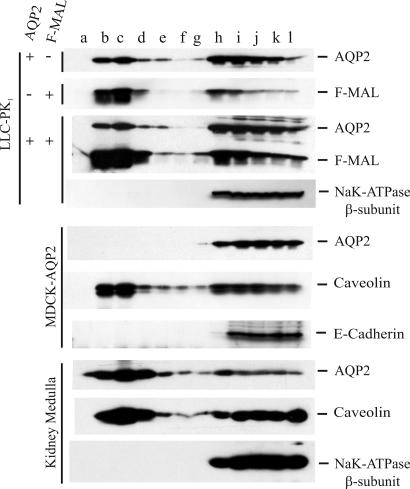
MAL is present in glycosphingolipid-enriched membranes and is thought to play a role in the incorporation of cargo into these membrane subdomains (7, 8, 10, 11). Glycosphingolipid-enriched membranes are resistant to detergent extraction, and, therefore, we carried out detergent extraction and sucrose gradient flotation experiments to assess whether the introduction of MAL into LLC-PK1 cells induces incorporation of AQP2 into these so-called DRM. These membranes, in this case insoluble when lysed at 4°C in 1% Triton X-100, will float from the bottom to the top of a nonlinear sucrose gradient during centrifugation. Immunoblots of gradient fractions reveal that MAL is mainly present in the early low-density fractions, suggesting DRM association (Fig. 3). In the MAL-negative LLC-PK1 cells, AQP2 is partially present in DRM. Unexpectedly, coexpression of MAL with AQP2 in these cells did not result in an increased DRM association of AQP2. The β-subunit of the NaK-ATPase is exclusively present in the late fractions, indicating that proteins not associated with DRM are completely solubilized in these experiments. Forskolin treatment also has no effect on the DRM association of AQP2 in the presence or absence of MAL (SI Fig. 8A). These data suggest that MAL does not recruit AQP2 into DRM. MDCK cells express MAL endogenously (10, 11) and also deliver AQP2 to the apical plasma membrane upon forskolin treatment (13). Surprisingly, AQP2 is not associated with DRM in these MDCK cells, either without or with forskolin treatment (Fig. 3 and SI Fig. 8B). Caveolin, but not E-cadherin, was found in DRM in the MDCK cells, indicating that DRM were extracted from these cells and behaved appropriately in the gradient. Because of this discrepancy, we investigated the raft association of AQP2 derived from native kidney material ex vivo, revealing that AQP2 is partially detected in DRM in the kidney (Fig. 3). Overall, these data indicate that MAL interacts with AQP2 but is not required for its apical delivery or involved in its DRM association.
Fig. 3.
MAL is not involved in the DRM association of AQP2. LLC-PK1 cells stably expressing F-MAL, AQP2, or both (indicated on the left), MDCK-AQP2 cells, and rat kidney inner medulla membranes were lysed in 1% Triton X-100 at 4°C. Insoluble membranes (DRM) were separated by flotation during sucrose-gradient centrifugation. Fractions (a–l) taken from the top to the bottom of the gradient were subjected to immunoblotting with antibodies specific for the membrane proteins indicated on the right. These data are representative of three independent cell line experiments and two independent experiments with kidney membranes.
MAL Increases the Steady-State Surface Expression of AQP2 in LLC-PK1 Cells.
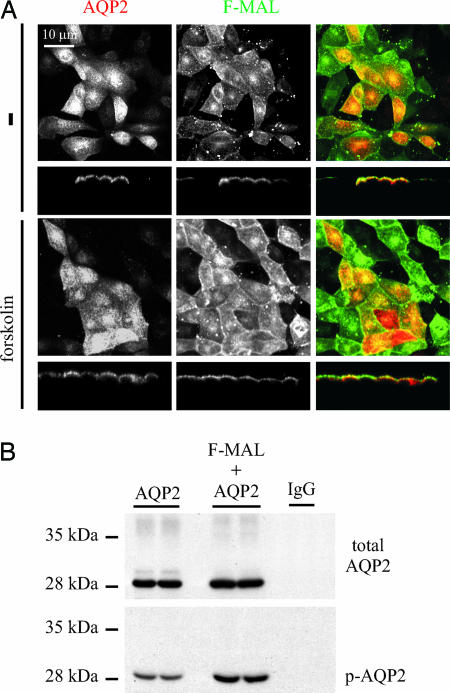
To our surprise, coexpression of AQP2 with MAL in LLC-PK1 cells resulted in apical expression of AQP2 that is independent of forskolin stimulation (Fig. 4A). This behavior is in marked contrast to that manifested by MAL-negative LLC-PK1 cells, in which AQP2 is retained in intracellular compartments in the absence of forskolin stimulation (Fig. 2). Apical cell surface biotinylation was carried out to confirm this increase in apical plasma membrane expression (SI Fig. 9). Because the translocation of AQP2 to the apical plasma membrane requires phosphorylation of AQP2 at S256, we wondered whether the increase in surface expression of AQP2 caused by coexpression with MAL coincided with an increase in the phosphorylation of AQP2. At similar expression levels of total AQP2, more phosphorylated AQP2 was detected in the LLC-PK1 cells that coexpress MAL and AQP2 than in those expressing AQP2 alone (Fig. 4B). Quantification of the signals revealed a 2-fold increase in phosphorylation of AQP2 when coexpressed with MAL (the ratio of phosphorylated AQP2 to total AQP2 is 0.36 ± 0.03 and 0.16 ± 0.01 for the cells with and without ectopic MAL expression, respectively; P < 0.05; n = 3). Thus, MAL expression in LLC-PK1 cells results in an increase in the steady-state presence of AQP2 in the apical plasma membrane, which correlates with an increase in the phosphorylation of AQP2.
Fig. 4.
MAL increases the surface expression and phosphorylation state of AQP2. (A) LLC-PK1 cells stably coexpressing AQP2 and F-MAL were not treated (−) or treated with forskolin for 45 min. Subsequently, immunofluorescence was performed for AQP2 and Flag (F-MAL). Images of AQP2 (Left) and Flag-MAL (Center) were collected with a confocal laser-scanning microscope and merged (Right). For both untreated and forskolin-treated cells, x–y sections (Upper) and x–z sections (Lower) are shown. (Scale bar: 10 μm.) n = 3. (B) LLC-PK1 cell lines expressing AQP2 alone or in combination with F-MAL were grown to confluence in duplicate and lysed in CHAPS in the presence of phosphatase inhibitors. AQP2 was immunoprecipitated with AQP2 antibodies. These immunoprecipitates and a control lane with AQP2 antibodies (IgG) were immunoblotted for AQP2 (total AQP2) or phosphorylated AQP2 (p-AQP2). Marker bands are indicated on the left by their molecular masses in kilodaltons.
MAL Decreases the Internalization of AQP2.
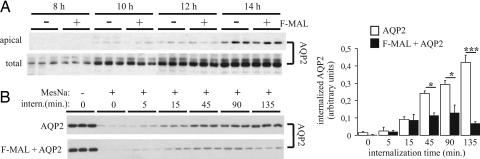
MAL was suggested to play a role in the initial biosynthetic delivery of several newly synthesized membrane proteins, including HA, gp80, and gp114, to the apical cell surface (10, 11). Therefore, we wished to investigate whether MAL would also increase the rate or extent of apical delivery of newly synthesized AQP2. We used a tetracycline-repressible expression system to induce expression of AQP2 once the cells reached confluence, because nonconfluent cells do not exhibit the capacity to express AQP2 at the cell surface (14). Several stably transfected cell lines revealed AQP2 expression between 8 and 12 h of induction (data not shown). One of these cell lines was supertransfected with the Flag-MAL-encoding construct or was stably mock-supertransfected with empty vector. In representative cell lines of both types, AQP2 expression was induced for 8, 10, 12, or 14 h. Subsequently, apical cell surface biotinylation revealed that AQP2 is delivered to the apical cell surface after 10 h of induction, with increasing amounts arriving after 12 and 14 h (Fig. 5A). However, at very similar expression levels, no difference in the rate or extent of apical delivery is observed between the cell lines with and without Flag-MAL. These data indicate that the increased steady-state apical surface expression of AQP2 in the presence of MAL is not caused by increased delivery of newly synthesized AQP2.
Fig. 5.
MAL decreases the rate of endocytosis of AQP2. (A) A representative LLC-PK1 cell line with tetracycline-repressible AQP2 expression was supertransfected with the Flag-MAL (F-MAL) expression vector (+) or with the empty vector (−). The cell lines were grown to confluence in the presence of tetracycline. AQP2 expression in both cell types was induced by incubation in tetracycline-free medium for the indicated time points. Subsequently, apical cell surface proteins were biotinylated, and recovered biotinylated proteins were immunoblotted for AQP2 (apical). Total AQP2 expression was assessed by immunoblotting total cell lysates (total). The image is representative of results obtained with three independent cell lines per transfection. (B) LLC-PK1 cell lines expressing AQP2 alone or in combination with F-MAL were grown to confluence and biotinylated from the apical side. Subsequently, cells were allowed to internalize (intern.) proteins for the indicated times. The remaining surface-accessible biotin was then stripped with MesNa where indicated (MesNa, +). Biotinylated proteins were recovered and immunoblotted for AQP2. Each experimental condition was examined in triplicate. Internalization of AQP2 (in arbitrary units ± SEM) was determined from three independent experiments by densitometry of the biotinylated AQP2 signals and by comparison to an AQP2 dilution series, and normalized to the amount of AQP2 detected in nonstripped samples (−). *, P < 0.05; ***, P < 0.01.
To examine whether MAL affects the internalization of surface AQP2, the MAL-negative and MAL-transfected LLC-PK1-AQP2 cells were subjected to an internalization experiment after forskolin treatment. Therefore, cells were labeled at the apical cell surface with cleavable biotin, returned to 37°C to allow internalization, and treated with the membrane-impermeant reducing agent MesNa. Internalized biotinylated proteins are protected from biotin cleavage by the reducing agent and thus are recovered. After 45–135 min of internalization, more biotinylated AQP2 is protected from the MesNa strip in MAL-negative than in MAL-transfected cells (Fig. 5B). These data suggest that MAL decreases the internalization of AQP2 in LLC-PK1 cells, accounting for the increased surface expression of this protein.
Discussion
We find that association with MAL slows the internalization of AQP2, resulting in an increase in the population of this water channel that is present in the apical plasma membrane. Interestingly, MAL-overexpressing mice develop dramatically amplified renal epithelial cell apical membranes as well as cysts in the tubular system of the kidney (15). It is tempting to hypothesize that MAL exerts these effects by slowing the internalization of apical membrane surface, leading to a net increase in apical surface area. The consequent stabilization of transport proteins, including AQP2, at the apical surfaces of renal epithelial cells of these transgenic animals may account for the fluid secretion and accumulation associated with cyst formation.
Membrane domains enriched in cholesterol and glycosphingolipids are implicated in governing apical sorting of a number of membrane proteins (16). MAL is incorporated into these glycosphingolipid-enriched membranes (as defined by detergent inextractability and low buoyant density) and is thought to direct sorting by recruiting apical cargo into these membrane microdomains (10, 11). In LLC-PK1 cells, however, AQP2 is already partially present in DRM in the absence of MAL, and coexpression with MAL does not increase this association. MDCK cells express MAL endogenously (10, 11) and sort AQP2 to the apical plasma membrane. Surprisingly, AQP2 was not at all associated with DRM in MDCK cells. These data suggest that MAL is not important for incorporation of AQP2 into DRM and that apical sorting of AQP2 is independent of DRM association. A number of proteins, including rhodopsin, the gastric H, K-ATPase, and several peptidases are also delivered to the apical surface by DRM-independent mechanisms (17–19), indicating that there exist alternative pathways for the sorting of apical cargo.
Interestingly, MAL appears to associate preferentially with AQP2-S256D, which mimics phosphorylated AQP2, as compared with AQP2-S256A, which mimics nonphosphorylated AQP2. Additionally, the presence of MAL increased the fraction of AQP2 expressed in LLC-PK1 cells that is phosphorylated, correlating with its elevated steady-state surface expression. This increase in the steady-state phosphorylation of AQP2 may also explain why forskolin stimulation has no additive effect on the amount of AQP2 that coimmunoprecipitates with MAL (SI Fig. 7). Altogether, these data indicate that MAL increases the surface expression of AQP2 by effecting a decrease in its internalization and that this coincides with an increase in the steady-state level of phosphorylation of AQP2.
Presumably MAL accomplishes the decrease in net AQP2 internalization through some combination of a decrease in the endocytosis of surface AQP2 and an increase in the exocytosis of AQP2 from recycling endosomes, although the precise mechanism remains to be studied. One possible explanation for the increased surface expression of AQP2 in the presence of MAL is based on the possibility that MAL binds to and stabilizes the phosphorylated form of AQP2. Phosphorylation of AQP2 is associated with its surface expression, and, thus, preventing dephosphorylation might reduce its internalization, either by reducing AQP2 endocytosis or by stimulating its insertion into the apical plasma membrane. Conversely, the dephosphorylation of AQP2 may occur only after internalization. According to this scenario the observed increase in the fraction of phosphorylated AQP2 would be attributable to its stabilized presence at the cell surface. Furthermore, it was recently observed that short-chain ubiquitination of AQP2 enhances its endocytosis (2). Whether MAL association attenuates ubiquitination of AQP2 or inhibits the endocytosis of ubiquitinated AQP2 is a subject for further study.
The influenza HA protein has also been shown to interact with MAL (11). Using antisense techniques to down-regulate MAL expression levels in MDCK cells, the authors concluded that this treatment causes a decrease in DRM association of HA and results in a decreased accumulation of HA at the apical surface. These investigators concluded that MAL plays an important role in ensuring the delivery of HA to the cell surface. Their experiments, however, did not measure directly the rates of HA surface delivery and/or endocytosis (20, 21). It is also worth noting that both LLC-PK1 cells (this study) and the Caco-2 human colon carcinoma cell line (22) express no endogenous MAL, although the influenza HA protein is still sorted to the apical plasma membrane in these cell types (23, 24). Although MAL has been implicated in the overall apical delivery of secretory proteins and transmembrane proteins (10, 20, 21), it seems difficult to reconcile completely this model with the biochemical behaviors of many apical-targeted proteins and with the rather limited expression profile of the MAL protein. Many polarized sorting processes are thought to be initiated within the confines of the trans-Golgi network. If MAL plays a direct and general role in this process then it might be expected to recruit its apical cargo into the DRM microdomains with which it is associated. However, approximately half of the proteins that have been shown to exhibit a MAL requirement for their apical sorting are not found in these DRM (20, 21). On the basis of our results, therefore, we suggest that MAL's primary role in apical sorting may be attributable either to the stabilization and retention of MAL partner proteins at the apical surface after their initial apical delivery or to the accelerated return to the apical surface of MAL partner proteins that have been endocytically internalized.
Body water homeostasis is critically dependent on water reabsorption in the kidney. The mechanism of regulated water reabsorption in the renal collecting duct relies on the recycling of AQP2 between intracellular vesicles and the apical plasma membrane. AQP2 probably recycles continually between these two organelles (3), and a change in the rate of any of those two opposing processes will be accompanied by a change in the steady-state localization of AQP2. Recent data suggest that arginine vasopressin (AVP) increases the AQP2 population in the apical plasma membrane by decreasing the rate of endocytosis of phosphorylated AQP2, rather than stimulating its translocation to the plasma membrane (25). Therefore, MAL likely modulates the internalization of AQP2 by decreasing its endocytosis. This mechanism could play an important role in mediating this channel's apical redistribution in response to arginine vasopressin and, consequently, in the regulation of body water homeostasis.
Materials and Methods
Constructs, Cell Culture, and Transfection.
Flag-tagged MAL was amplified by using the primers F-5′-GCCCAAGCTTATGGACTACAAGGACGACGATGACAAGGCCCCCGCAGCGGCGACGGGG-3′ (Flag epitope in bold, HindIII site underlined) and R-5′-GCGCCTCGAGTTATGAAGACTTCCATCTGAT-3′ (KpnI site underlined). The 520-bp product obtained was digested with HindIII and KpnI and subcloned into the respective sites of pCDNA3.1+-Hygro (Invitrogen, Carlsbad, CA). Constructs encoding WT-AQP2, AQP2-S256A, and AQP2-S256D have been described previously (12). Inducible expression was realized by using a tTA-regulated bicistronic expression vector (26). The entire cDNA of AQP2 was amplified by using the forward primer 5′-GGAATTCTGCAGCCCTGCCGCCACCATGTGGGAGCTCCGCTCCGC-3′, containing an EcoRI restriction site (underlined) and an optimal Kozak sequence (bold), and a reverse primer containing a NotI restriction site. Subsequently, this PCR fragment was subcloned into the EcoRI/NotI sites of pNRTIS-21, and this construct was stably transfected into LLC-PK1 cells. Repression of AQP2 expression was obtained by using 100 ng/ml tetracycline.
All cells were grown in α-MEM supplemented with 10% FBS and 2 mM l-glutamine. COS-7 cells were transfected with Lipofectamine 2000 (Invitrogen). LLC-PK1 cells were transfected by using Ca3(PO4)2 as described earlier (27). Stable LLC-PK1 clones were selected with 1.6 mg/ml G418 or 0.8 mg/ml hygromycin B for eight passages. At least three clonal cell lines per transfection were analyzed.
Immunoblotting and Semiquantification of Signals.
PAGE, Western blotting, and chemiluminescence of the resultant membranes were performed as described (27). All primary antibodies were diluted in 1% nonfat dried milk in TBS-T in the following dilutions: (M2) Flag (1:4,000; Sigma, St. Louis, MO), rabbit or guinea pig AQP2 (1:3,000 or 1;1,000) (27), rabbit phosphorylated AQP2 (1:100) (28), monoclonal Na,K-ATPase β-subunit (1:500) (29), rabbit caveolin (1:10,000; Transduction Laboratories, San Jose, CA), and rat antibodies to E-cadherin (1:5,000; Sigma). Quantification of signals was performed by scanning densitometry. Short exposures manifesting signals within the linear range were used, and a 2-fold dilution series was included to serve as an internal standard curve. P values between two variables were calculated by Student's t test between multiple variables using ANOVA.
Immunoprecipitation.
COS-7 cells from one well of a six-well plate or confluent LLC-PK1 cells from one 10-cm-diameter dish were lysed (4% CHAPS/0.1% BSA/150 mM NaCl/25 mM Hepes). Six rat kidney medullae were homogenized in homogenization buffer (300 mM sucrose/25 mM imidazole/1 mM EDTA). Nuclei and unbroken cells were removed by centrifugation at 4,000 × g, after which membranes were isolated by centrifugation at 200,000 × g and solubilized in lysis buffer. Nonsolubilized material was pelleted, and the supernatant was precleared of endogenous Igs. The cell lysates and solubilized kidney membranes were subjected to immunoprecipitations (using 0.5 μg of M2 Flag antibody) or 4 μl of rabbit MAL antibodies (Abcam, Cambridge, U.K.), respectively.
Immunohistochemistry.
Anesthetized water-deprived rats were fixed by perfusion with 1% paraformaldehyde lysine phosphate after which their kidneys were removed. Frozen sections of these kidneys were subjected to sequential incubations with the following antibodies: rabbit MAL (1:50), Alexa Fluor 488-conjugated anti-rabbit (1:100; Molecular Probes, Eugene, OR), guinea pig anti-AQP2 (1:100), and Alexa Fluor 594-conjugated anti-guinea pig (1:100; Molecular Probes) by using TSA Direct (PerkinElmer). The sections were dehydrated with methanol and embedded in Mowiol.
Immunofluorescence in LLC-PK1 cells was performed as in MDCK cells (27), except for M2 Flag incubations (1:200 dilution). For these, the cells were permeabilized for 30 min in permeabilization buffer (0.3% saponin/0.1% BSA in PBS), and antibodies were diluted in isotonic goat serum dilution buffer (16% goat serum/0.3% saponin in PBS). Then the filters were mounted on slides in Vectashield (Vector Laboratories, Burlingame, CA).
Cell Surface Biotinylation and Internalization Experiments.
These experiments were performed as described (2). Briefly, cells grown to confluence on permeable supports were biotinylated with cell-impermeable NHS-SS-biotin from the apical side. For the internalization experiments, cells were returned to 37°C, after which surface-bound biotin was removed by using cell-impermeable sodium mercaptoethanol (MesNa). Next, biotinylated proteins were recovered by using immobilized streptavidin and immunoblotted.
Triton Insolubility and Flotation Experiments.
These experiments were performed as described (30) with minor modifications. Cells were grown to confluence, scraped, and pelleted. Membranes from kidney inner medullae of two rats were prepared as for the immunoprecipitation protocol. The rest of the experiment was performed on ice in a 4°C cold room. The cell pellet or medulla membranes were resuspended in 1 ml of ice-cold TNE (10 mM Tris, pH 7.4/150 mM NaCl/1 mM EDTA), mixed with 1 ml of 2% Triton X-100 in TNE, and incubated for 30 min. Next, the lysate was passed five times through a 22-gauge needle, mixed with 2 ml of 80% (wt/vol) sucrose in TNE, placed on the bottom of an ultracentrifuge tube (for an SW40-TI rotor), subsequently overlaid with 8 ml of 30% sucrose in TNE and 2 ml of 5% sucrose in TNE, and centrifuged at 200,000 × g for 16 h. Sequential 1-ml fractions were taken from the top and analyzed by immunoblotting.
Supplementary Material
Acknowledgments
We thank Dr. Søren Nielsen (Water and Salt Research Center and Institute of Anatomy, University of Aarhus, Aarhus, Denmark) for the generous gift of the antibody recognizing phosphorylated AQP2 and Vanathy Rajendran and Sue-Ann Mentone for technical assistance. This work was supported by National Institutes of Health Grants DK17433 and GM072614 (to M.J.C.), European Molecular Biology Organization Grant ALTF-155-2001 (to E.-J.K.), and The Netherlands Organization for Scientific Research Grant 916.36.122 (to E.-J.K.).
Abbreviations
- AQP
aquaporin
- MDCK
Madin–Darby canine kidney
- DRM
detergent-resistant membrane.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708023104/DC1.
References
- 1.Nielsen S, Kwon TH, Frokiaer J, Agre P. J Intern Med. 2007;261:53–64. doi: 10.1111/j.1365-2796.2006.01760.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Proc Natl Acad Sci USA. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun TX, van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Am J Physiol. 2002;282:F998–F1011. doi: 10.1152/ajprenal.00257.2001. [DOI] [PubMed] [Google Scholar]
- 4.Chou CL, Knepper MA, Hoek AN, Brown D, Yang B, Ma T, Verkman AS. J Clin Invest. 1999;103:491–496. doi: 10.1172/JCI5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouraud S, Laera A, Calamita G, Carmosino M, Procino G, Rossetto O, Mannucci R, Rosenthal W, Svelto M, Valenti G. J Cell Sci. 2002;115:3667–3674. doi: 10.1242/jcs.00053. [DOI] [PubMed] [Google Scholar]
- 6.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Am J Physiol. 2001;281:F1092–F1101. doi: 10.1152/ajprenal.0091.2001. [DOI] [PubMed] [Google Scholar]
- 7.Kim T, Fiedler K, Madison DL, Krueger WH, Pfeiffer SE. J Neurosci Res. 1995;42:413–422. doi: 10.1002/jnr.490420316. [DOI] [PubMed] [Google Scholar]
- 8.Frank M, van der Haar ME, Schaeren-Wiemers N, Schwab ME. J Neurosci. 1998;18:4901–4913. doi: 10.1523/JNEUROSCI.18-13-04901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons K, Ikonen E. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 10.Cheong KH, Zacchetti D, Schneeberger EE, Simons K. Proc Natl Acad Sci USA. 1999;96:6241–6248. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puertollano R, Martin-Belmonte F, Millan J, de Marco MC, Albar JP, Kremer L, Alonso MA. J Cell Biol. 1999;145:141–151. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Balkom BWM, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PMT. J Biol Chem. 2002;277:41473–41479. doi: 10.1074/jbc.M207525200. [DOI] [PubMed] [Google Scholar]
- 13.Deen PMT, Rijss JPL, Mulders SM, Errington RJ, van Baal J, van Os CH. J Am Soc Nephrol. 1997;8:1493–1501. doi: 10.1681/ASN.V8101493. [DOI] [PubMed] [Google Scholar]
- 14.Ausiello DA, Hall D. J Biol Chem. 1981;256:9796–9798. [PubMed] [Google Scholar]
- 15.Frank M, Atanasoski S, Sancho S, Magyar JP, Rulicke T, Schwab ME, Suter U. J Neurochem. 2000;75:1927–1939. doi: 10.1046/j.1471-4159.2000.0751927.x. [DOI] [PubMed] [Google Scholar]
- 16.Verkade P, Simons K. Histochem Cell Biol. 1997;108:211–220. doi: 10.1007/s004180050161. [DOI] [PubMed] [Google Scholar]
- 17.Dunbar LA, Aronson P, Caplan MJ. J Cell Biol. 2000;148:769–778. doi: 10.1083/jcb.148.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Lu D, Sadler JE. J Biol Chem. 1999;274:1596–1605. doi: 10.1074/jbc.274.3.1596. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Belmonte F, Arvan P, Alonso MA. J Biol Chem. 2001;276:49337–49342. doi: 10.1074/jbc.M106882200. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Belmonte F, Puertollano R, Millan J, Alonso MA. Mol Biol Cell. 2000;11:2033–2045. doi: 10.1091/mbc.11.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Marco MC, Martin-Belmonte F, Kremer L, Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA, Alonso MA. J Cell Biol. 2002;159:37–44. doi: 10.1083/jcb.200206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ. J Biol Chem. 1998;273:26862–26869. doi: 10.1074/jbc.273.41.26862. [DOI] [PubMed] [Google Scholar]
- 24.Riento K, Kauppi M, Keranen S, Olkkonen VM. J Biol Chem. 2000;275:13476–13483. doi: 10.1074/jbc.275.18.13476. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Am J Physiol. 2004;286:F233–F243. doi: 10.1152/ajprenal.00179.2003. [DOI] [PubMed] [Google Scholar]
- 26.Tenev T, Bohmer SA, Kaufmann R, Frese S, Bittorf T, Beckers T, Bohmer FD. Eur J Cell Biol. 2000;79:261–271. doi: 10.1078/S0171-9335(04)70029-1. [DOI] [PubMed] [Google Scholar]
- 27.Deen PMT, Van Balkom BWM, Savelkoul PJ, Kamsteeg EJ, Van Raak M, Jennings ML, Muth TR, Rajendran V, Caplan MJ. Am J Physiol. 2002;282:F330–F340. doi: 10.1152/ajprenal.0168.2001. [DOI] [PubMed] [Google Scholar]
- 28.Christensen BM, Zelenina M, Aperia A, Nielsen S. Am J Physiol. 2000;278:F29–F42. doi: 10.1152/ajprenal.2000.278.1.F29. [DOI] [PubMed] [Google Scholar]
- 29.Gottardi CJ, Caplan MJ. J Biol Chem. 1993;268:14342–14347. [PubMed] [Google Scholar]
- 30.Brown DA, Rose JK. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.