Abstract
Homeostatic control of volume within the alveolar spaces of the mammary gland has been proposed to involve a feedback system mediated by serotonin signaling. In this article, we describe some of the mechanisms underlying this feedback based on studies of a human normal mammary epithelial cell line (MCF10A) and mouse mammary epithelium. Mammary serotonin was elevated during lactation and after injection of 5-hydroxytryptophan (5-HTP). The genes encoding the serotonin reuptake transporter (SERT) and the type 7 serotonin receptor (5-HT7) were expressed in human and mouse mammary epithelial cells, and serotonin caused a concentration-dependent increase of cAMP in MCF10A cells. Mouse and human mammary epithelial cells formed polarized membranes, in which tight junction activity was monitored. Treatment of mammary epithelial membranes with serotonin receptor antagonists increased their transepithelial electrical resistance (TEER). Antagonist and agonist effects on TEER were mediated by receptors on the basolateral face of the membranes. Our results suggest a process in which serotonin accumulates in the interstitial fluid surrounding the mammary secretory epithelium and is detected by 5-HT7 receptors, whereupon milk secretion is inhibited. One mechanism responsible for this process is serotonin-mediated opening of tight junctions, which dissipates the transepithelial gradients necessary for milk secretion.
Keywords: lactation, MCF10A, milk protein, serotonin receptor, serotonin transporter
Volume homeostasis within a closed, fluid-filled space is a common physiological problem for multicellular organisms, and maintenance of volume–space homeostasis typically requires two kinds of feedback networks: those that involve multiple organs and those that are tissue-autonomous. Disturbances of volume–space homeostasis contribute to pathologies such as hypertension, glaucoma, hydrocephaly, cystic fibrosis, mastitis, and polycystic kidney disease. In the case of milk filling in the mammary glands, homeostatic regulation of volume is a major practical problem for the dairy industry. Also, nursing mothers who need to collect breast milk when they are away from their babies and mothers of premature infants confront major problems associated with milk homeostasis (1).
In the breasts, volume–space homeostasis is achieved by complex interactions among signals that travel through neuroendocrine pathways (2) and signals strictly within the local environment of the glands (3, 4). Waves of prolactin (PRL) and oxytocin are secreted in response to nipple stimulation during suckling, causing milk synthesis, secretion, and ejection by acting on the secretory epithelium and myoepithelium. This neuroendocrine feedback loop is the primary extrinsic mechanism of homeostasis during lactation, and the specific components of this systemic feedback loop have been known for many years. In contrast to this well known neuroendocrine system, the intrinsic homeostatic mechanisms in the breasts remain largely unknown.
Milk synthesis is regulated within the alveolar units of the breast so as to control the degree of alveolar distension in the short term, and adjust milk secretion to the demands of the offspring, in the long term. Correspondingly, each functional unit within the gland is regulated by local signals. In the normal lactogenic cycle, the milk stasis-associated feedback is essential between bouts of nursing or artificial milking in dairy animals. Studies done in goats showed that physical distension alone was insufficient to cause inhibition of milk secretion. The implication of this line of studies was that some chemical signal, in addition to gland distention, was necessary for feedback control of milk secretion (5–7). However, neither the identity of a chemical signal nor a mechanism of action has been definitively proved.
Our laboratory previously reported evidence of serotonin [5-hydroxytryptamine (5-HT)] mechanisms in mouse mammary gland development and homeostasis (8). The gene for tryptophan hydroxylase 1 (TPH1) was highly induced in mammary glands of hyperprolactinemic mice. TPH1 mRNA was elevated during pregnancy and lactation in mice, and 5-HT was detected in the mammary epithelium and in milk. Milk protein gene expression was suppressed by 5-HT in prolactin (PRL)-stimulated mouse primary mammary epithelial cells (mPMEC). Correspondingly, treatment of mammary gland explants and mPMEC with methysergide (MS), a broad-spectrum antagonist of 5-HT receptors (5-HT1,2,5,6,7), resulted in increased expression of milk protein genes and accumulation of secretory material and lipid globules. These results implied that the gene for TPH1 was part of a negative-feedback mechanism that was induced in response to stimulation by PRL, and was not part of the stimulatory pathway activated by PRL.
One of the physiological events that is essential for lactation is the establishment and maintenance of epithelial tight junctions (9, 10). The full functional integrity of epithelial tight junctions is established shortly after parturition, and they remain closed throughout lactation (9, 11–13). Changes in breast fluid composition attributable to tight junction closure (i.e., altered sodium, potassium, and serum protein concentrations) occur after parturition in women, indicating that the same general process of tight junction closure occurs in the human breast (14). Conversely, opening of tight junctions has been suggested to be one of the early events in mammary involution.
Addressing the serotonin mechanisms associated with mammary homeostasis, we describe findings that shed light on three points: (i) the existence of serotonergic mechanisms in human as well as mouse mammary cells, (ii) the effect of 5-HT receptor activity on mammary epithelial tight junctions, and (iii) the receptors involved in mammary 5-HT signaling. These studies suggest new ways to understand (and potentially control) volume–space homeostasis in the breast.
Results
Mammary Gland Tissue Levels of 5-HT.
Mice were used to test the proposed functional role of TPH1 expression in the mammary glands. In a previous study, our laboratory (8) showed that TPH1 gene expression was elevated in pregnant and lactating mice compared with nonpregnant controls, but the study did not demonstrate whether there were changes in 5-HT per se. Consequently, we measured the tissue level of 5-HT in mouse mammary glands. We found an ≈2.5-fold increase in tissue 5-HT content in lactating mice compared with virgin controls (Table 1). The increase of 5-HT in lactation was similar to the change of TPH1 gene expression during lactation (8). To test whether TPH1 was rate-limiting for mammary 5-HT, we injected mice with 5-hydroxytrpytophan, the immediate downstream product of TPH1. There was an acute increase of >5-fold in the tissue level of 5-HT (Table 1).
Table 1.
Tissue concentrations of 5-HT in mouse mammary glands
| Mice | Tissue (5-HT), ng/g tissue | P value vs. control |
|---|---|---|
| Control | 36.60 ± 0.50 (2) | — |
| Lactating | 99.82 ± 12.79 (6) | <0.05 |
| 5-HTP-treated | 194.70 ± 26.10 (2) | <0.01 |
Extracts from mammary glands of normal virgin control, days 10–11 lactating, and 100 mg/kg body weight 5-HTP-injected female mice were assayed by HPLC/EC. Values are expressed as means ± SE relative to tissue wet weights. Data were compared by ANOVA, with Newman–Keuls test for differences among means.
5-HT Signaling Proteins in Mammary Epithelial Cells.
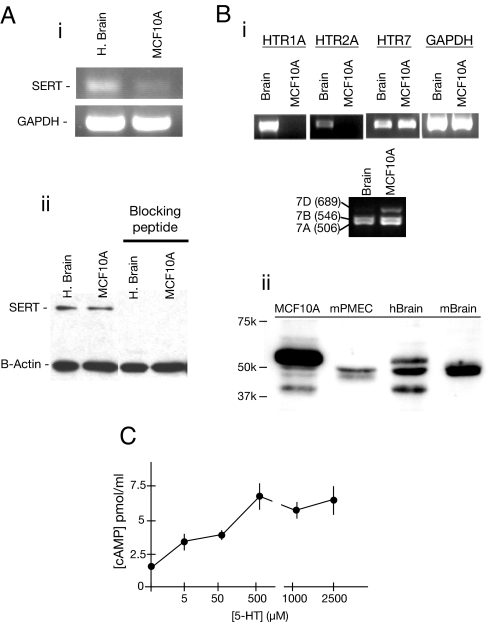
Human MCF10A mammary epithelial cells expressed not only the gene encoding TPH1 (8), but also the serotonin transporter (SERT) and the type 7 serotonin receptor protein (5-HT7) (Fig. 1). These tissue-restricted components, along with two ubiquitous enzymes (aromatic amine decarboxylase and monoamine oxidase), establish a human mammary epithelial cell line that is capable of 5-HT biosynthesis, metabolism, reuptake, and receptor-mediated response.
Fig. 1.
Expression of SERT and 5-HT receptors in mammary epithelial cells. (A) RT-PCR and Western blot for SERT in human brain and MCF10A cell extracts. (B) (i) RT-PCR for 5-HT receptors in positive control brain and MCF10A cell extracts, along with the housekeeping gene (GAPDH). Selective primers distinguished the expression of three known human splice variants of HTR7. (ii) Western blot for 5-HT7 in human and mouse mammary cells, with brain from each species as a positive control. (C) cAMP accumulation in MCF10A cells in response to 5-HT. Averages (±SEM) are of cells from four dishes. The positive control of cells incubated with 2 μM forskolin was measured to be 12.6 ± 0.6 pmol/ml. The result is representative of three experiments.
SERT mRNA was identified in mouse mammary gland tissue and primary epithelial cells (data not shown), and SERT mRNA and protein were identified in a human MCF10A cell line (Fig. 1A). These findings suggest that SERT is likely to be physiologically relevant in mammary epithelium.
The HTR7 transcript is processed by alternative splicing to produce three variants in humans (HTR7a, HTR7b, and HTR7d), which encode proteins with similar signaling properties (15). MCF10A cells generate all three HTR7 mRNAs. The predominant isoform of the 5-HT7 protein in MCF10A cells was the ≈52,000 Mr isoform (Fig. 1B). Mouse mammary epithelial cells also expressed 5-HT7, with the same apparent molecular size as the major mouse brain isoform. To establish functional effector coupling of 5-HT receptors to Gs, we measured cAMP accumulation in MCF10A cells. The response to 5-HT indicated the presence of a Gs-coupled 5-HT signal-transduction pathway in MCF10A cells, with concentration-dependent increases in cAMP (Fig. 1C).
Mammary Epithelial Cells Form Functionally Polarized Membranes on Permeable Supports.
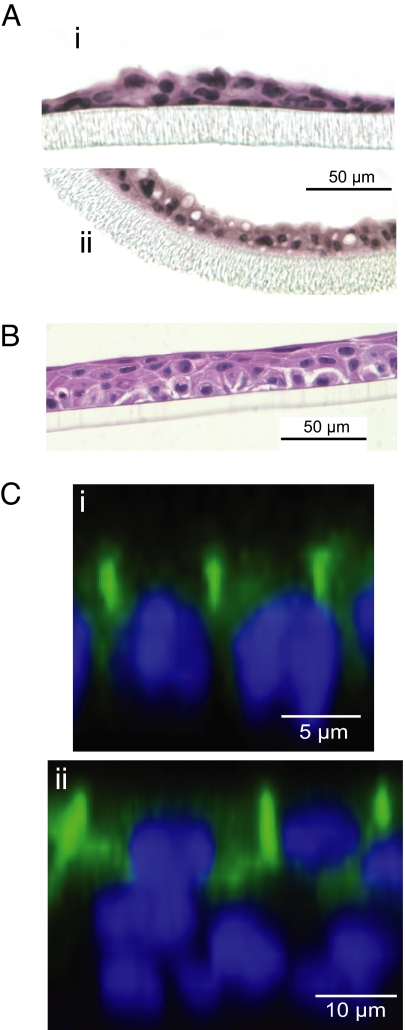
The morphology of mouse and human mammary cells cultured on Transwell permeable supports was studied by conventional histology and immunostaining (Fig. 2). The mPMEC under control conditions (no lactogenic hormones) had small cytoplasmic volumes and comprised a thin pseudostratified membrane. After stimulation with lactogenic hormones, the cells had much larger cytoplasmic volumes, including fat globules (Fig. 2A). Human MCF10A mammary epithelial cells grown on Transwell permeable supports generated membranes composed of a densely packed basal cell layer (proximal to the supporting membrane) overlain by an apical cell layer consisting of a mixture of squamous and columnar cells (Fig. 2B). Addition of lactogenic hormones did not cause any obvious or robust changes in the morphology of the MCF10A membranes (data not shown), although there were modest effects on transepithelial resistance (discussed later). Immunostaining for occludin in mPMEC cultures showed localization of tight junctions and polarization of the cell membranes (Fig. 2C). In MCF10A cells, occludin immunostaining identified the apical cell layer as having distinct accumulations of this tight junction protein in association with the lateral plasma membranes. This localization suggests that the apical layer performs the function of an epithelial barrier. The basal cells, in contrast, did not have membrane-associated occludin.
Fig. 2.
The morphology of mammary epithelial cells on Transwell permeable cell culture supports. (A) Sections through membranes of mPMEC in control medium (i) or lactogenic medium (ii), showing stimulation of lipid globules in lactogenic medium. (B) MCF10A cells grown on polyester membrane (untreated control). (C) Confocal images of mPMEC (i) and MCF10A (ii) cells immunostained for occludin (green) with nuclei (blue). Little or no occludin stain was observed in the basal layer.
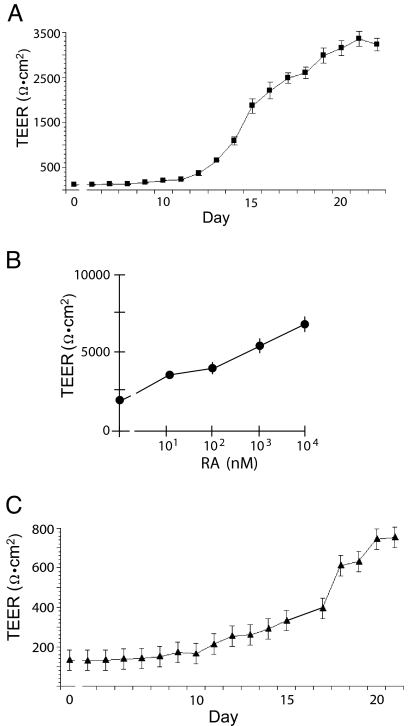
We monitored tight junction functionality by measuring transepithelial electrical resistance (TEER). A characteristic profile for TEER of MCF10A membranes (Fig. 3A) started at a baseline of ≈120 Ωcm2, which was not different from Transwell permeable membranes without cells. After a lag period of several days, the TEER increased rapidly to a plateau maximum of ≈3,000 Ωcm2. We tested whether either of two important differentiation factors, PRL or all-trans retinoic acid (RA), altered the development of TEER in MCF10A membranes. Addition of PRL to the culture medium resulted in small, but consistent, increases in the maximal TEER of the MCF10A membranes (data not shown). In contrast to the small effect of PRL, all-trans RA caused a large concentration-dependent increase of TEER in the MCF10A membranes (Fig. 3B), indicating a role for retinoid receptor signaling in the differentiation of the MCF10A membranes. Similar TEER profiles were observed with mPMEC (Fig. 3C). However, in contrast to MCF10A cells, the mPMEC required PRL in the medium for the TEER increase.
Fig. 3.
TEER measurements of MCF10A cells and mPMEC grown on transwell membranes. Each value is the average of three or four wells (±SEM) of TEER (y axis, note different scales). (A) Typical profile of MCF10A cell membranes. (B) Concentration-response curve (molar:log10) for TEER of MCF10A membranes treated for 48 h with all-trans RA. The treatment was started 24 h before predicted peak TEER. (C) TEER profile of mPMEC in lactogenic medium (1 μg·ml−1 PRL and 1 μM dexamethasone).
Effects of Serotonergic Agents on TEER.
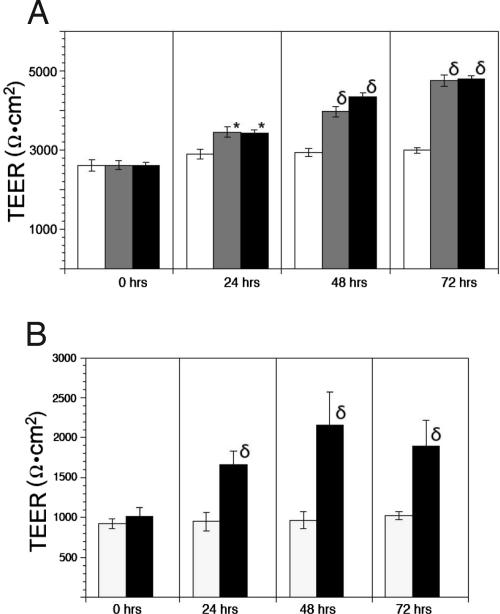
MCF10A cells were cultured on permeable supports with medium containing methysergide (MS) or metergoline (MG), two broad-spectrum 5-HT receptor antagonists, and the effects on TEER were determined. Drugs were added to both chambers of the cultures beginning early on the plateau of the TEER profile. MCF10A cells treated with either MS or MG recorded increases in TEER compared with controls by 24 h (Fig. 4A), and the drug effect increased to 33% above controls at 48 h and 60% above controls at 72 h. The effect of MG was similar in Transwell cultures of mPMEC (Fig. 4B). Consistent with the receptor expression profile, when we tested a variety of selective antagonists in MCF10A and mPMEC membranes, only SB 269,970, a 5-HT7 antagonist, caused an increase of TEER (data not shown).
Fig. 4.
Elevation of TEER in response to 5-HT receptor antagonists. Values are graphed as means ± SEM (n = 3) for each measurement time point. Drugs were added at 10 μM concentration after the 0 time point measurement. (A) MCF10A cells treated with growth medium (open columns), MS (shaded columns), or MG (filled columns). (B) mPMEC treated with medium only (open columns) or MG (filled columns). *, P < 0.05 vs. control; δ, P < 0.001 vs. control.
Serotonergic Agents Act by the Basal Membrane.
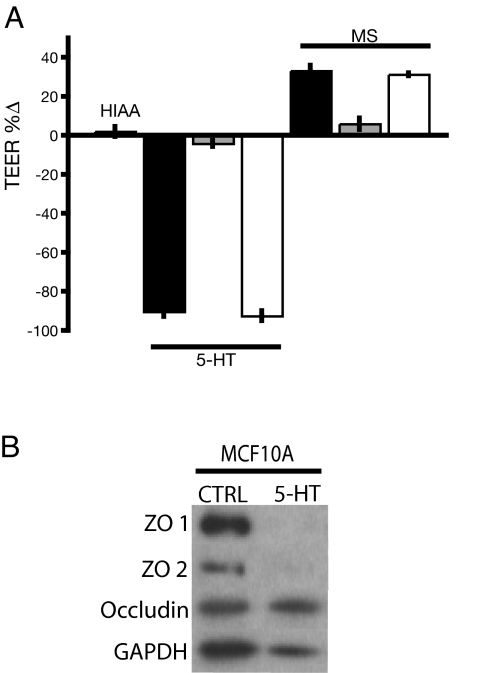
The Transwell cell culture inserts allowed us to test whether drugs administered to the apical or basal compartments had different effects on TEER. An inactive analog, 5-hydroxyindole acetic acid, had no effect on TEER. The natural agonist, 5-HT, was added to either both chambers of the Transwell dishes or each chamber individually, causing a dissipation of TEER when it was present in both chambers or the basal chamber only (Fig. 5A). This result indicates that 5-HT receptors were located on the basolateral membranes. The antagonist, MS, had the same effect when added to only the basal compartment as when added to both, but there was no effect of MS added to the apical compartment only (Fig. 5A). The topological restriction of the drug effects to the basal surface demonstrated that the effect was specific (i.e., receptor-mediated), and that the relevant receptors were located on the basal, but not the apical, face of the membranes. Moreover, Western blot analysis from 5-HT-treated transwell cultures showed that the decline in TEER after treatment with 5-HT was associated with a marked decrease in levels of tight junction scaffolding proteins ZO1 and ZO2 (Fig. 5B), which are known to be essential for tight junction formation and maintenance.
Fig. 5.
Mechanism of tight junction regulation in MCF10A cells. (A) Basal polarity of 5-HT7 receptor functionality in MCF10A membranes. Data are expressed as the percentage change of experimental vs. control values (±SEM) measured 48 after drug addition. Bars are labeled according to the drugs applied: HIAA, 2.5 mM 5-hydroxyindole acetic acid; 5-HT, 2.5 mM serotonin. Drug treatment was given on both sides (filled bar), apical side only (shaded bar), and basal side only (open bar). (B) Western blot for tight junction proteins with GAPDH as loading control on MCF10A transwell lysates in control (untreated) and 0.75 mM 5-HT (treated) conditions.
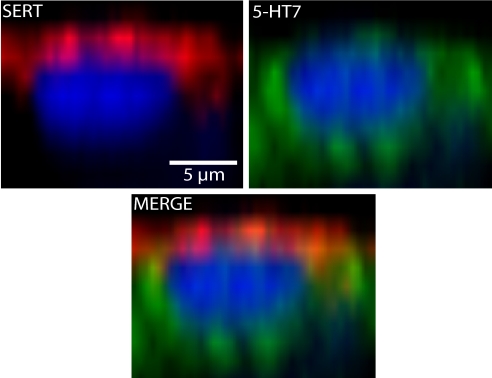
We immunostained for 5-HT7 and SERT proteins in transwell-cultured MCF10A cells to determine the localization of each. In Fig. 6, a Z-plane image of a representative apical MCF10A cell shows that SERT staining was tightly associated with the apical surface and not detectable in the basal cell layer. In contrast, 5-HT7 was localized to the basolateral membranes of the apical cells. The location of immunoreactive 5-HT7 is consistent with the pharmacological evidence, showing that functional 5-HT receptors were facing the basal chamber.
Fig. 6.
Immunolocalization of SERT (red) and 5-HT7 (green) in MCF10A membranes. Representative Z-plane cross-sectional images of a representative apical cell. Note that the SERT and 5-HT7 immunoreactivity were largely nonoverlapping, with SERT associated with the apical and 5-HT7 associated with the basolateral membranes.
Discussion
The current studies were initiated after our laboratory reported that 5-HT was involved in mammary gland development and homeostasis (8). The initial finding was that the gene for TPH1 was highly induced in PRL-stimulated mammary glands, and 5-HT was detected in the mammary epithelium and in milk. Milk protein gene expression was suppressed by 5-HT in PRL-stimulated mPMEC. Correspondingly, treatment of mammary gland explants and mPMEC with MG, a 5-HT receptor antagonist, caused increased expression of milk protein genes and accumulation of secretory material and lipid globules. We also showed that TPH1-deficient mice were hypersensitive to PRL in the mouse mammary gland and the level of 5-HT is correlated with the level of TPH1, confirming that TPH activity is rate-limiting for 5-HT synthesis in the mammary gland.
Although MCF10A cells are immortalized and have some abnormal features, they are untransformed and capable of forming structures that can serve as good models of normal functions of the mammary epithelium (16, 17). When MCF10A cells are grown on permeable membrane supports, we observed a basal layer of cells in contact with the membrane that was overlain by an apical layer of well polarized cells. Occludin was associated with tight junctions along the boundaries of the apical cell layer, implying that the apical mix of squamous and columnar cells creates an epithelial barrier in membranes composed of MCF10A cells. The basal MCF10A cell layer on permeable supports appears to play a role roughly analogous to basal epithelial cells in vivo (18).
The 5-HT reuptake protein (SERT) was identified in MCF10A cells, primary cells, and mammary tissue. Furthermore, SERT was found to be specifically located on the apical membrane of the epithelial barrier forming cells in transwell cultures. The presence of SERT in the mammary epithelial cells and its specific localization in transwell cultures raise questions about the likely function of SERT in the mammary gland. The presence of SERT also raises the possibility that selective serotonin reuptake inhibitors, such as fluoxetine, could have effects on the breast or milk. A physiological role for mammary SERT is currently speculative, and the clinical evidence regarding the effects of selective serotonin reuptake inhibitors during breastfeeding is limited and ambiguous (19, 20).
5-HT Receptors in Mammary Epithelium.
We observed increased TEER in MCF10A cells and/or PMEC treated with either of two broad-spectrum 5-HT receptor antagonists, MS and MG. This finding naturally led to the question of which receptor types are expressed and functional in mammary epithelium. 5-HT exerts its effects through a family of receptors classified into seven subfamilies (5-HT1–7) based on structural, functional, and pharmacological properties. 5-HT receptors regulate cellular activities by secondary messengers like cAMP and phospholipid metabolites. Therefore, it was important to know the expression of 5-HT receptors in mammary epithelial cells. We found that the gene encoding 5-HT7 was expressed in both human and mouse mammary epithelial cells. Three splice variants of 5-HT7 have been identified in humans, which only differ in the C terminus (15). The 5-HT7 receptor, which couples to Gs, mediated a 5-HT effect on cAMP accumulation in MCF10A cells. However, we did not find a simple relationship between cAMP signaling and tight junctions (data not shown). The failure to find a direct correspondence between cAMP and tight junction regulation implies that other effectors that have been linked to 5-HT7 signaling, such as MAP kinases or Rho GTPases (21), may contribute to mammary 5-HT7 signaling.
Tight Junctions and Mammary Homeostasis.
Given the importance of tight junction integrity for lactation (9, 10), we focused much of our physiological characterization of 5-HT signaling on the regulation of epithelial tight junctions. As a mediator of milk stasis-induced involution, 5-HT has effects on a variety of aspects of lactating mammary epithelial cells, including milk protein synthesis, fat globule synthesis, fluid and solute secretion, and cell survival (8). Many of these effects, including the effects on tight junctions, could be indirect. Identifying the relevant signaling pathways allows for the design of experiments to sort out the hierarchy of 5-HT regulatory effects.
To the extent that the in vitro systems used for our studies serve as valid models for the mammary epithelium in vivo, we can infer that 5-HT receptors involved in controlling TEER are located on the basolateral membranes of luminal cells in the mammary gland. This conclusion is supported by the pharmacological observation that serotonergic drugs act by the basal compartment and the morphological observation that 5-HT7 receptors accumulate in the basal aspect of the luminal cells.
We propose that the local feedback on milk synthesis is mediated by the discharge of 5-HT into the luminal interstitium, which implies that 5-HT is either secreted across the basolateral membranes into the interstitium or that apically secreted 5-HT leaks out of the milk space and is detected by 5-HT receptors. Although these are not mutually exclusive mechanisms, they are functionally different and should be testable as alternatives. It is possible that 5-HT secretion directly into the interstitium, along with an increase of 5-HT synthesis, could explain the observed feedback effects of 5-HT. However, we previously showed that 5-HT is present in milk (8), implying that it is secreted apically and, therefore, would enter the interstitium by leaking through the tight junction barrier. It is conceivable that the induction of 5-HT synthesis, combined with increasing leakage from the milk space, would deliver a robust signal for feedback inhibition.
The findings in these studies provide evidence of molecular, cellular, and tissue mechanisms that constitute a previously unknown physiological system for homeostasis in a glandular luminal space. The mechanisms provide a basis for addressing several practical and theoretical problems in mammary gland biology that relate to the short-term homeostasis of milk yield in human mothers or dairy species. We do not yet know how these mechanisms impact long-term aspects of mammary biology, such as normal development and the pathogenesis of breast cancers, nor do we know whether other glandular spaces could be regulated by analogous mechanisms.
Methods
Cell Culture.
MCF10A (American Type Culture Collection, Manassas, VA) cells were cultured in complete growth medium (DMEM:F12, supplemented with 5% horse serum, 10 μg/ml insulin, 20 ng/ml EGF, 0.5 μg/ml hydrocortisone, 1 units/ml penicillin, 0.1 mg/ml streptomysin, 0.25 μg/ml amphotericin B, and 2 mM l-glutamine). For measurement of transepithelial resistance, the MCF10A cells were seeded at a high-plating density (105 cells per cm2) on clear polyester Transwell permeable supports (Corning, Corning, NY) in growth medium. Drugs were added to the culture media when TEER reached a plateau (2–3 weeks after seeding).
Primary mouse mammary epithelial cells were isolated as described (8). Cells were maintained in medium containing DMEM:F12 (supplemented with 1% FBS, 10 ng/ml EGF, 10 μM insulin, 0.5 units/ml penicillin, 50 μg/ml streptomysin, 0.25 μg/ml amphoterecin B with or without 1 μg/ml prolactin, and 1 μM dexamethasone). TEER was measured by using an epithelial volt-ohm meter (World Precision Instruments, Sarasota, FL). Media were changed daily throughout the course of the experiments.
Assay of Tissue 5-HT.
C57BL/6J mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities, and the studies were performed under protocols approved by the Institutional Animal Care and Use Committee. The animals were young adults (3–6 months of age) maintained with food (breeder chow) and water available ad libitum. Tissues (no. 4 mammary glands) were removed after killing the animals by CO2 asphyxiation, and the mammary glands were quickly frozen and stored at −80°C. Tissues were extracted with five volumes/wet weight of 0.2 M perchloric acid and centrifugation at high speed. The supernatants were assayed after being collected carefully with a syringe to avoid contamination from either the pellet or overlying fat layer. HPLC with electrochemical detection was performed as described (8).
Reverse Transcription Polymerase Chain Reaction.
Total RNA was isolated from cell pellets by using Tri Reagent, treated with DNase1 (Promega, Madison, WI), and extracted and precipitated by using a standard phenol/chloroform extraction method. Two micrograms of total RNA was reverse transcribed and amplified, followed by PCR amplification by using specific primers designed online using “Biology Workbench” (http://workbench.sdsc.edu). All products were validated by DNA sequencing. Primers specific for the human HTR7 isoforms were from ref. 22.
Western Blotting and Immunostaining.
Proteins isolated from cells at 80–100% confluency or from transwell cultures were used for Western blotting. Human and mouse brain tissue lysates (Imgenex, San Diego, CA) were used as positive controls. The blots were incubated in optimized concentrations of primary antibodies (5-HT7, Imgenex; SERT, ATS, San Diego, CA; ZO1, ZO2, and occludin, Zymed Laboratories, South San Francisco, CA; GAPDH, Sigma–Aldrich, St. Louis, MO) overnight at 4°C. Proteins were visualized with HRP-conjugated secondary antibodies (Sigma–Aldrich) as appropriate.
Cells grown on Transwell permeable supports were fixed in 4% paraformaldehyde for histological analyses. Sections were permeabilized in 0.1% Trition X-100, incubated in borate buffer [80 mM boric acid, 20 mM sodium borate (pH 8.5)] overnight at 75°C. The sections were incubated in primary antibodies overnight at 4°C (5HT7, Imgenex; occludin, Zymed Laboratories; SERT, ATS). Images were collected by using a Zeiss LSM510 confocal microscope, and Z stacks were reconstructed three dimensionally by using the Zeiss LSM Image Software version 3.5 (Carl Zeiss, Thornwood, NY).
Acknowledgments
We thank K. Neiport, M. Mistry, M. Reilly, and L. Merk for technical contributions. This work was supported by National Institutes of Health Grant DK52134, Department of the Army Grant BC052576 (to N.D.H.), and Predoctoral Fellowship HD007463 (to A.M.M.).
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HT7
type 7 serotonin receptor protein
- MG
metergoline
- MS
methysergide
- RA
retinoic acid
- TEER
transepithelial electrical resistance
- TPH
tryptophan hydroxylase
- PRL
prolactin
- SERT
serotonin transporter.
Footnotes
The authors declare no conflict of interest.
References
- 1.Riordan J, Auerbach KG. Breastfeeding and Human Lactation. Boston: Jones and Barlett Publishers; 1999. p. xxii.p. 874. [Google Scholar]
- 2.Kacsoh B. Endocrine Physiology. New York: McGraw–Hill; 2000. p. 741. [Google Scholar]
- 3.Knight CH, Peaker M, Wilde CJ. Rev Reprod. 1998;3:104–112. doi: 10.1530/ror.0.0030104. [DOI] [PubMed] [Google Scholar]
- 4.Wilde CJ, Prentice A, Peaker M. Proc Nutr Soc. 1995;54:401–406. doi: 10.1079/pns19950009. [DOI] [PubMed] [Google Scholar]
- 5.Henderson AJ, Peaker M. J Physiol. 1984;351:39–45. doi: 10.1113/jphysiol.1984.sp015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson AJ, Peaker M. Q J Exp Physiol. 1987;72:13–19. doi: 10.1113/expphysiol.1987.sp003039. [DOI] [PubMed] [Google Scholar]
- 7.Wilde CJ, Addey CV, Casey MJ, Blatchford DR, Peaker M. Q J Exp Physiol. 1988;73:391–397. doi: 10.1113/expphysiol.1988.sp003155. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M, Bailey JP, Nieport KM, Walther DJ, Bader M, et al. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DA, Neville MC. J Mammary Gland Biol Neoplasia. 1998;3:233–246. doi: 10.1023/a:1018707309361. [DOI] [PubMed] [Google Scholar]
- 10.Itoh M, Bissell MJ. J Mammary Gland Biol Neoplasia. 2003;8:449–462. doi: 10.1023/B:JOMG.0000017431.45314.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stelwagen K, Davis SR, Farr VC, Eichler SJ, Politis I. J Dairy Sci. 1994;77:2994–3001. doi: 10.3168/jds.S0022-0302(94)77240-4. [DOI] [PubMed] [Google Scholar]
- 12.Stelwagen K, Farr VC, Davis SR, Prosser CG. Am J Physiol. 1995;269:R848–R855. doi: 10.1152/ajpregu.1995.269.4.R848. [DOI] [PubMed] [Google Scholar]
- 13.Stelwagen K, Farr VC, McFadden HA, Prosser CG, Davis SR. Am J Physiol. 1997;273:R379–R386. doi: 10.1152/ajpregu.1997.273.1.R379. [DOI] [PubMed] [Google Scholar]
- 14.Neville MC. Clin Perinatol. 1999;26:251–279. [PubMed] [Google Scholar]
- 15.Heidmann DE, Metcalf MA, Kohen R, Hamblin MW. J Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- 16.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 17.Debnath J, Muthuswamy SK, Brugge JS. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 18.DiRenzo J, Signoretti S, Nakamura N, Rivera-Gonzalez R, Sellers W, Loda M, Brown M. Cancer Res. 2002;62:89–98. [PubMed] [Google Scholar]
- 19.Gentile S. CNS Drugs. 2005;19:623–633. doi: 10.2165/00023210-200519070-00004. [DOI] [PubMed] [Google Scholar]
- 20.Eberhard-Gran M, Eskild A, Opjordsmoen S. CNS Drugs. 2006;20:187–198. doi: 10.2165/00023210-200620030-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, Dityateva G, Schachner M, Voyno-Yasenetskaya TA, Ponimaskin EG. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahé C, Bernhard M, Bobirnac I, Keser C, Loetscher E, Feuerbach D, Dev KK, Schoeffter P. Br J Pharmacol. 2004;143:404–410. doi: 10.1038/sj.bjp.0705936. [DOI] [PMC free article] [PubMed] [Google Scholar]