Abstract
Chromosome territory (CT) organization and chromatin condensation have been linked to gene expression. Although individual genes can be transcribed from inside CTs, some regions that have constitutively high expression or are coordinately activated loop out from CTs and decondense. The relationship between epigenetic marks, such as DNA methylation, and higher-order chromatin structures is largely unexplored. DNMT3B mutations in immunodeficiency centromeric instability facial anomalies (ICF) syndrome result in loss of DNA methylation at particular sites, including CpG islands on the inactive X chromosome (Xi). This allows the specific effects of DNA methylation on CTs to be examined. Using fluorescence in situ hybridization, we reveal a differential organization of the human pseudoautosomal region (PAR)2 between the CTs of the X and Y in normal males and the active X (Xa) and the Xi in females. There is also a more condensed chromatin structure on Xi compared with Xa in this region. PAR2 genes are relocalized toward the outside of the Y and Xi CTs in ICF, and on the Xi, we show that this can extend to genes distant from the site of DNA hypomethylation itself. This reorganization is not simply a reflection of the transcriptional activation of the relocalized genes. This report of altered CT organization in a human genetic disease illustrates that DNA hypomethylation at restricted sites in the genome can lead to more extensive changes in nuclear organization away from the original site of epigenetic change.
Keywords: X inactivation, Y chromosome
To fully understand the orchestration of gene expression, it is necessary to understand how chromatin is spatially organized within the cell nucleus. In particular, the position of a gene with respect to its chromosome territory (CT) and the chromatin condensation of its genomic region have been linked to gene activation and repression (1). Although individual genes can be transcribed from inside of CTs (2), some regions that have constitutively high gene expression (3, 4) or are subject to coordinate gene activation during development (5, 6) or differentiation (7) or in response to physiological stimuli (8) locate at the edge or outside of their CT. This level of nuclear reorganization is often accompanied by a visible level of chromatin decondensation (5, 6, 9). The precise function, if any, of repositioning toward the outside of CTs remains unclear (1), but it may allow for genes to access a nuclear environment enriched in the components of the transcription (10) and/or mRNA-processing machinery (4, 11, 12) and so enhance the efficiency of transcription.
The interplay between this level of higher-order chromatin organization and epigenetic mechanisms that act at primary levels of chromatin structure, such as DNA methylation, is a little-explored area. In plants, changes in the architecture of CTs can be induced by treatment with inhibitors of DNA methylation (13). However, the genome-wide demethylation that follows 5-azacytidine treatment or genetic deficiency in DNA methyltransferases makes it difficult to investigate the direct effects of DNA methylation on chromosome organization at specific loci. Mutations in the DNA methyltransferase DNMT3B in immunodeficiency centromeric instability facial anomalies (ICF) syndrome patients (14) lead to loss of DNA methylation at specific genomic sites (15, 16). Prominent sites of hypomethylation in ICF are satellite DNAs in the juxtacentromeric heterochromatin at chromosome regions 1qh, 16qh, and 9qh, and aberrant association of the satellite II-rich 1qh and 16qh regions has been seen in nuclei of ICF cells (17). Sites on the female inactive X chromosome (Xi), especially CpG islands, are also hypomethylated in ICF syndrome. This does not reflect a chromosome-wide defect in gene inactivation on Xi. In female ICF cells, the Xi still forms a Barr body, is associated with the XIST noncoding RNA, and mostly replicates late (15). However, two genes, SYBL1 and G6PD, do show abnormal escape from X chromosome inactivation (XCI) in female ICF cells (16). SYBL1 is located in the pseudoautosomal region (PAR)2 of the long arms of the human X and Y chromosomes. In this region, genes that are subject to, and that escape, XCI are closely linked. Of the two PAR2 genes (SPRY3 and SYBL1) that are subject to XCI, only SYBL1 is modified by DNA methylation (at its CpG island) on both Xi and the Y of male cells. In ICF, hypomethylation of the SYBL1 CpG island is associated with abnormal expression of both the Xi- and Y-linked SYBL1 alleles (14). The inactivation of SPRY3 is independent of DNA methylation and is unaltered in ICF cell lines (Fig. 1 and Table 1) (14, 18).
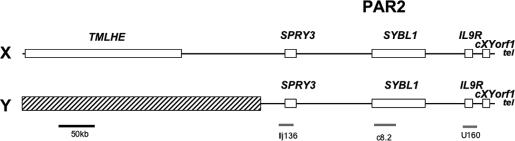
Fig. 1.
Genomic map of PAR2 of the human sex chromosomes. Map of the PAR2 on Xq and Yq showing, from the centromere to the telomere (tel), the positions of the genes (open boxes), and the cosmid probes (gray lines) used in FISH experiments. The hatched box indicates the heterochromatin of Yq12.
Table 1.
Summary of the transcriptional behavior of the PAR2 genes on the Xi and Y chromosomes and the involvement of DNA methylation in their regulation in both WT and ICF cells
| Feature | Chromosome | Gene |
|
|---|---|---|---|
| SPRY3 (WT/ICF) | SYBL1 (WT/ICF) | ||
| Expression | Xi | −/− | −/+ |
| Y | −/− | −/+ | |
| DNA methylation | Xi | −/− | +/− |
| Y | −/− | +/− | |
Because the hypomethylation defect is restricted to a limited number of loci, ICF cells provide a unique opportunity to examine the specific effect of loss of DNA methylation on the organization of CTs. We used interphase FISH to examine the nuclear organization of PAR2 genes on the long arms of the human X and Y chromosomes (Fig. 1). We show that there is a differential organization of the human PAR2 among the CTs of the X and Y in normal males and the active X (Xa) and the Xi in females. We also provide direct evidence for a more condensed chromatin structure in the PAR2 region on Xi compared with Xa. In ICF, we show that PAR2 genes are relocalized toward the outside of the Y and Xi CTs and that, on Xi, this relocalization is not limited to genes that are themselves directly subject to hypomethylation and is not just a passive consequence of their transcriptional activation. This report of altered CT organization in a human genetic disease illustrates that the influence of DNA hypomethylation at restricted sites in the genome is far more extensive than previously imagined, which might, in part, explain why genes whose expression is dysregulated in ICF are often found not to be the direct targets of DNA hypomethylation themselves (19).
Results
Differential Nuclear Organization of the PAR2 Region on the X and Y.
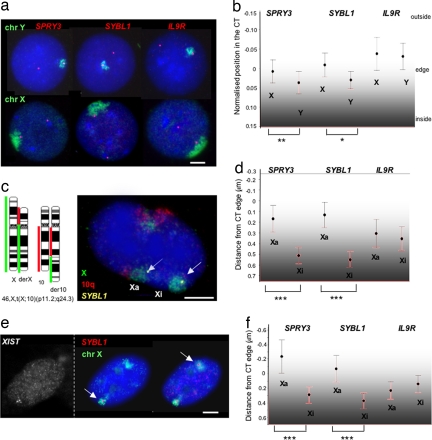
SYBL1 and SPRY3 are transcribed only on the X in male cells and the Xa in female cells (20, 21). The silent Xi and Y alleles of both genes are associated with repressive histone marks, but only SYBL1 silencing is associated with DNA methylation (Table 1) (14, 18). We first used 2D FISH to compare how the differentially expressed alleles of SPRY3 and SYBL1 are organized relative to territories of the X and Y in cells from a normal male (Fig. 2a). 2D FISH allows for fast and automated image analysis and can reveal differences in CT organization, even though it exaggerates the absolute distances measured as compared with 3D FISH and so may not fully reflect in vivo structure (6, 22). On the Y, we found that most (≈70%) SPRY3 and SYBL1 hybridization signals are inside, or just at the edge (±0.2 μm), of the CT, consistent with the silent state of these genes. There were <5% of signals located far (>0.6 μm) outside of the CT of the Y [see supporting information (SI) Fig. 5a]. In contrast, although most of the hybridization signals for the expressed X-linked alleles are still localized inside of the CT, 24–36% are located outside. This may represent the proportion of alleles that are actively transcribing, because SYBL1 primary transcript is observed in ≈35% of cells by RNA FISH (data not shown). This differential organization of the PAR2 region on the X and Y is reflected in the average position of the genes relative to each CT. The X-linked alleles are significantly closer to the edge of the CT than the Y-linked alleles (Fig. 2b).
Fig. 2.
Nuclear organization of PAR2 in male and female cells. (a) DNA FISH, using Y (top row) or X (bottom row) chromosome paints (green) together with cosmid probes (red) for SPRY3, SYBL1, and IL9R on methanol/acetic acid (MA)-fixed nuclei of normal male LCLs. Nuclei were counterstained with DAPI (blue). (Scale bar, 5 μm.) (b) Mean [±95% confidence interval (CI)] position for each allele of the three PAR2 genes relative to the edge of the X and Y CT in MA-fixed cells. Because the territory of the X chromosome is larger than that of the Y (reflecting their different DNA contents), the position of each cosmid probe signal was normalized relative to the radius of a circle of area equal to that of the corresponding CT. The significance of the differences in position between X- and Y-linked alleles is indicated. *, P ≤ 0.01; **, P ≤ 0.005. (n ≥ 70.) (c) Four-color DNA FISH, using chromosome paints for 10q (red) and the X (green), together with an SYBL1-specific probe (yellow, arrow) on nuclei of the female fibroblast cell line GM07693 that carries a balanced translocation, 46,X,t(X;10). The normal chromosome is Xi. The karyogram of the normal and derivative chromosomes X and 10 is shown at Left. (Scale bar, 5 μm.) (d) Mean (±95% CI) position (in micrometers) for each allele of the three PAR2 genes relative to the edge of the Xa and Xi CT in MA-fixed GM07693 cells. The significance of the differences in position between alleles is indicated. ***, P ≤ 0.000. (n ≥ 65.) (e) (Left) RNA FISH with XIST probe. (Right) DNA FISH, using SYBL1 and X chromosome paint. Shown are pictures of the same nucleus on two different planes of focus in the z axis to visualize the 3D position of SYBL1 probe (Scale bar, 5 μm.). (f) Mean (±95% CI) position (in micrometers) for each allele of the three PAR2 genes relative to the edge of the Xa and Xi CT in paraformaldehyde-fixed WI38 cells. ***, P ≤ 0.000. (n ≥ 50.)
IL9R is weakly expressed in our EBV-transformed lymphoblast cells (data not shown). However, in cell types where it is expressed, it is transcribed from both sex chromosomes (23). Consistent with this, both alleles of IL9R have the same location relative to the X and Y CTs (Fig. 2b), which suggests that transcribed PAR2 alleles are located more externally to the CT than inactive ones.
Differential Nuclear Organization of the PAR2 Region Between the Xa and Xi.
This correlation, between the differential CT position of X- and Y-linked SPRY3 and SYBL1 alleles and their expression, could reflect inherent differences between the organization of the X and Y chromosomes or could be part of a more general allele-specific silencing pathway for these genes. In the latter case, there may also be differential positioning of SPRY3 and SYBL1 relative to the CTs of the Xa and Xi in female cells.
To address this question, we first exploited a female fibroblast cell line (GM07693) with a balanced translocation, 46,X,t(X;10) (p11.2;q24.3) (Fig. 2c). X inactivation in this cell line is skewed (>95:5) such that the normal X chromosome is the Xi, and expression of SPRY3 and SYBL1 originates from the translocated X (14, 24). We distinguished the Xa and Xi in 2D FISH, using paints for both 10q and the X (Fig. 2c). Thirty to 40% of the SPRY3 and SYBL1 hybridization signals are located at the edge or outside of the Xa territory, whereas this is seen for only 4–8% of signals on the Xi (SI Fig. 5b). Therefore, both SPRY3 and SYBL1 have significantly different positions relative to the Xa and Xi CTs (Fig. 2d). Differential nuclear organization is restricted to the two PAR2 genes with allele-specific expression profiles. Signals for IL9R, which is not expressed in these fibroblast cells, occupy similar positions relative to the edge of the Xa and Xi CTs.
To exclude any effect of the translocation on the organization of the Xa in GM07693 and to confirm our findings by 3D FISH in formaldehyde-fixed cells, we performed combined RNA/DNA FISH on a female cell line with a normal karyotype (WI38 fibroblasts), using RNA FISH for XIST to identify the Xi (Fig. 2e). In these cells, SPRY3 and SYBL1 are expressed, but IL9R is not transcribed (data not shown). We first analyzed individual CTs in their plane of focus, using the same scripts as for 2D FISH. This confirmed the more external localization of SPRY3 and SYBL1, but not IL9R, on the territory of the Xa compared with the Xi (Fig. 2f and SI Fig. 5c). In addition, we visually examined PAR2 nuclear organization in these cells on z-stacks taken at 0.2-μm intervals along the z axis and manually scored the alleles as inside or outside of the CT. In 80% of cell signals for SPRY3 and SYBL1, Xa alleles were more outside of the CT than their counterparts on Xi (n = 25). These data suggest that the location of PAR2 genes within the CT is not just a generalized feature of the organization of Xa, Xi, or Y but that it correlates to the specific regulation of gene expression.
The Xi Is More Condensed Than the Xa at PAR2.
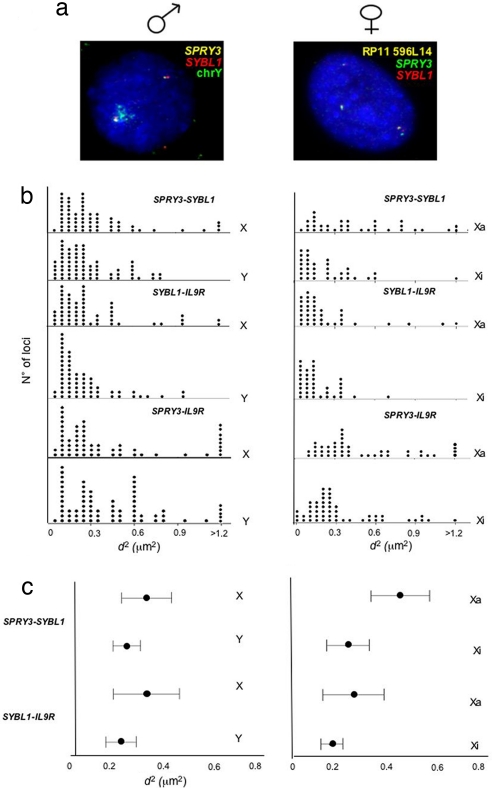
Movement away from CTs is often, but not always, accompanied by a visible increase in the interphase distance (d) between probe signals. Mean-squared interprobe distances (d2) are related to genomic separation, but differences in d2 for a constant genomic separation are thought to represent a decondensation of higher-order chromatin structure (5, 6, 9, 25). We used 2D FISH to measure d between SPRY3 and SYBL1, SYBL1 and IL9R, and SPRY3 and IL9R in both male and female (GM07693) cells. The translocated X (Xa) in GM07693 female cells was identified with a BAC probe from 10q25 (Fig. 3a). Differences in d2 between the X and Y in male cells were not statistically significant (Fig. 3 b and c), even though the PAR2 genes are differentially expressed and spatially organized between these two chromosomes. There is also no significant difference in the SYBL1–IL9R interprobe distances between the two X chromosomes of female cells. However, there is a significant (P < 0.000) increase in d2 between the SPRY3 and SYBL1 alleles of the Xa compared with those on the Xi, which is also confirmed by the measurement of the distance between SPRY3 and IL9R genes (Fig. 3 b and c). Therefore, there is decondensation of the chromatin on the Xa, specifically in the SPRY3–SYBL1 region.
Fig. 3.
Interphase chromatin condensation of PAR2. (a) FISH with probes for SPRY3 and SYBL1 in nuclei from male (Left) or female GM07693 (Right) cells. The X- and Y-linked alleles in male cells were distinguished by cohybridization with a Y chromosome paint. The Xa and Xi alleles in GM07693 cells were distinguished by hybridization with a chromosome 10 BAC (RP11 596L14). (b) Distribution of squared interphase distances (in square micrometers) measured between SPRY3 and SYBL1, SYBL1 and IL9R, and SPRY3 and IL9R probes on X and Y in male cells (Left) and Xa and Xi in female cells (Right). (n ≥ 57.) (c) Mean (±95% CI) squared interphase distances (in square micrometers) measured between SPRY3 and SYBL1 and SYBL1 and IL9R probes on X and Y in male cells (Left) and Xa and Xi in female cells. (n ≥ 57.)
Altered CT Organization of the PAR2 Region in ICF Syndrome.
Biallelic SYBL1 expression in two ICF cell lines, derived from male (PT5) and female (PT3) patients, accompanies reduced CpG methylation at its promoter. Additionally, all of the differential histone modifications tested are erased in the same region (14, 16, 26). By contrast, the silencing of SPRY3 Xi and Y alleles is unaltered in these ICF cells, consistent with the fact that DNA methylation is not involved in the inactivation of this gene (Table 1) (18).
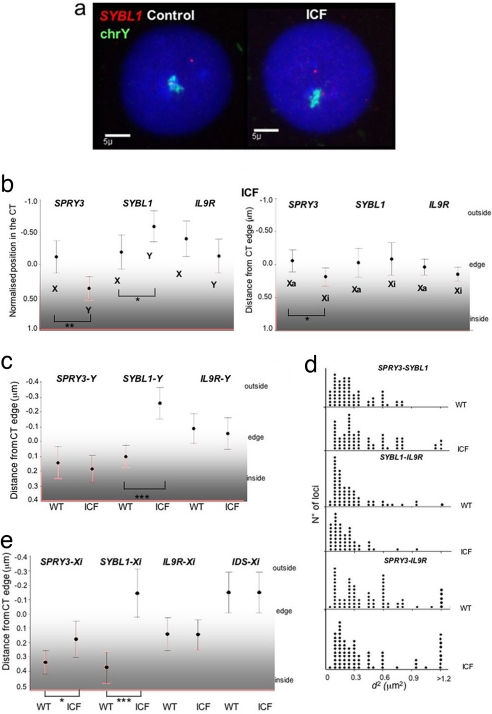
To determine whether the altered gene expression and DNA methylation of SYBL1 affects its nuclear organization, we determined the CT position of PAR2 alleles in ICF (PT5) lymphoblast cell lines (LCLs) by 2D FISH (Fig. 4a). Whereas SYBL1 signals are mainly inside or just at the edge of the Y territory in WT cells (Fig. 2b and SI Fig. 5a), in PT5 cells, >60% of signals localize far outside of the territory (Fig. 4a and SI Fig. 6a) and indeed seem to be even more external than the X-linked allele (Fig. 4b and SI Fig. 5d). Compared with WT cells, there is a significant relocalization of SYBL1, but not of SPRY3 and IL9R, to a position external to the Y territory in ICF cells (Fig. 4c). This relocalization is also accompanied by a significant (P = 0.001) increase in the interphase distances between SPRY3 and SYBL1 but not between SYBL1 and IL9R (P = 0.22) (Fig. 4d), similar to what we saw when comparing the X and Y or the Xa and Xi alleles in normal cells (Fig. 3). The distance measured between the SPRY3 and IL9R in normal and ICF cells is not significantly different.
Fig. 4.
PAR2 interphase organization in control and ICF male cells. (a) 2D FISH with probe for SYBL1 (red) and Y chromosome paint (green) in control (Left) and ICF (Right) male LCLs. (b) Mean (±95% CI) position (in micrometers) for each allele of the three PAR2 genes relative to the edge of the X and Y CT (Left) and Xa and Xi (Right) in ICF male LCLs and female fibroblasts. The significance of differences in position between alleles is indicated. *, P ≤ 0.01; **, P ≤ 0.005. (n ≥ 65.) (c) Mean (±95% CI) position (in micrometers) for SYBL1-Y, SPRY3-Y, and IL9R-Y alleles relative to the edge of the Y territory in WT and ICF cells. ***, P ≤ 0.000. (n ≥ 70.) (d) Distribution of squared interphase distances (in square micrometers) measured between SPRY3 and SYBL1, SYBL1 and IL9R, and SPRY3 and IL9R probes on Y chromosomes in WT and ICF cells. (n ≥ 50.) (e) Mean (±95% CI) of the position (in micrometers) for SYBL1-Xi, SPRY3-Xi, IL9R-Xi, and IDS-Xi alleles relative to the edge of the Xi territory in WT (WI38 for SYBL1, SPRY3, and IL9R and GM01871 for IDS) and ICF fibroblasts. ***, P ≤ 0.000. (n ≥ 50.)
Because expression of SYBL1 is also altered from the Xi in ICF, we analyzed its position within the Xi CT in control female fibroblasts (WI38) and fibroblasts from the PT3 ICF patient, using XIST RNA FISH before 3D DNA FISH to distinguish between the Xa and Xi. Compared with WT, there is significant relocalization of SYBL1 to the outside of the Xi CT in ICF cells (Fig. 4e and SI Fig. 6b) so that it occupies an average position similar to the Xa allele (Fig. 4b and SI Fig. 5e).
Reorganization of Xi and Y territories in ICF cells could be a direct effect of altered DNA methylation on higher-order chromatin structure, or it could be a downstream consequence of transcriptional activation of SYBL1. However, although there is no relocalization of SPRY3 detected within the Y territory of ICF cells, there is a relocalization of SPRY3 signals toward a more external position within the Xi territory in ICF cells, even though this gene remains transcriptionally silent (Fig. 4e) (P = 0.01).
These data reveal a more extensive effect of DNA hypomethylation on the organization of the Xi than is seen on the Y. It has been suggested that nearly all CpG-island associated genes on Xi may be hypomethylated in ICF (16). To determine whether there is a more generalized effect of DNA hypomethylation on the organization of the Xi CT, we examined the gene IDS that is located ≈6 Mb proximal of PAR2 in Xq28. IDS is hypomethylated but not expressed on Xi in PT3 ICF fibroblasts (16). Consistent with its localization in a gene-dense region of the human genome (3), in GM01871 control female fibroblasts, IDS is localized toward the outside of the Xi CT, even though it remains transcriptionally silent (Fig. 4e and SI Fig. 6c). There is no relocalization of IDS in ICF cells (P = 0.15). Therefore, DNA hypomethylation in ICF does not result in a general disorganization of the Xi CT.
Discussion
In this study, we have shown that nuclear organization depends on chromosomal context and function, not on DNA sequence per se. We found that the PAR2, which undergoes a relatively high rate of recombination between the X and the Y (27), is differently organized between the CTs of the X and Y in male cells (Fig. 2b) and between the Xa and Xi of female cells (Fig. 2 d and f). In normal male and female cells, CT organization correlates with the expression status of PAR2 alleles, with the expressed alleles located toward the outside of their respective CTs.
Our data, showing that active PAR2 alleles appear positioned toward the outside of the Xa CT and that their inactive counterparts on Xi are positioned well inside of that CT, are consistent with some other studies of Xi organization. A study of X-linked ANT2 (subject to XCI) and the PAR1 gene ANT3, which escapes inactivation, suggested a differential position between inactive and active alleles. The active alleles were located toward the edge of X chromosome CTs, whereas the inactive ANT2 allele was positioned toward the interior of the Xi CT (28). CT organization has also been linked to the initiation of XCI. Very early in mouse ES cell differentiation, genes that are yet to be inactivated are found outside of the XIST RNA domain that coats the territory of the Xi. They then become localized inside of the XIST domain as they inactivate (29). Even in differentiated mouse somatic cells, genes that escape XCI remained outside of the XIST domain (29). In contrast, it has been suggested in one study that protein-coding genes of the human Xi, including the PAR1 gene MIC2, tend to be arranged at the periphery of the CT, regardless of whether they do or do not escape XCI, with the internal volume of the Xi composed of noncoding repetitive DNA (30). Although we cannot comment on the distribution of repetitive DNA within the Xi CT, our data are consistent with the idea that inactivated alleles are located well within the Xi CT, whereas active alleles are generally located at or beyond the edge of the Xa CT (Fig. 2 d and f).
We also found evidence for differential chromatin condensation/interphase separation between the Xa and Xi (but not between the X and Y) specifically in the proximal part of PAR2 (SPRY3–SYBL1) (Fig. 3c). The appearance of the Barr body in the nucleus of female cells has led many to speculate that there might be increased chromatin condensation on the Xi (31). However, this idea was questioned by a study that reported equivalent volumes, and hence equivalent levels of overall chromatin condensation, of Xa and Xi (32). That study used X chromosome paints prepared from plasmid libraries and may have lacked the sensitivity to detect decondensed chromatin located at the periphery of the Xa. We conclude that there is likely differential chromatin condensation between some specific regions of Xa and Xi.
In male ICF cells, there is a relocalization to the outside of the Y CT of the PAR2 gene SYBL1, which is hypomethylated and transcriptionally activated. The adjacent Y-linked SPRY3, which remains silent, is unaltered in its nuclear organization in ICF (Fig. 4c). This differential reorganization is reflected in an increased interphase separation between Y-linked SYBL1 and SPRY3 alleles in ICF cells (Fig. 4d). This would indicate that the effects of DNA hypomethylation on Y chromosome organization are restricted to the immediate vicinity of the activated SYBL1 gene. By contrast, in female ICF cells, both SYBL1 and SPRY3 relocalize to the outside of the territory of the Xi, even though, as in males, only SYBL1 is subject to hypomethylation and transcriptional activation (Fig. 4e). Hence, in this case, DNA hypomethylation causes changes in the interphase organization of the Xi that extend far beyond the genes that are immediately subject to hypomethylation and transcriptional activation. The Xi-allele of SPRY3 appears to be moved toward the periphery of its CT in the absence of actual activation of gene expression, reaffirming that a gene's intra-CT position is not just a passive consequence of its transcriptional activation. Analysis of other regions of the human genome has suggested that the CT organization of a gene is a reflection of its transcriptional potential and not activation per se and also depends on the activity of its surrounding genomic regions (3, 4). In the case of the PAR2 on Xi and Y, the different effects of ICF-induced hypomethylation on CT reorganization could be due to the nature of the X- and Y-specific sequences just proximal of this PAR. Although the X-linked PAR2 immediately abuts the gene-rich Xq28 region, the Y-linked PAR2 is adjacent to the block of heterochromatin at Yq12, and this might restrict CT reorganization (Fig. 1). Elsewhere in the genome, altered CT organization encompassing extended regions around hypomethylated sequences may allow for the inappropriate transcriptional activation of relocated genes if the right transcription factor environment is available (33). This may explain the failure to detect DNA hypomethylation at many genes whose expression is dysregulated in ICF (19).
Materials and Methods
Cell Culture.
Cell lines used in this study were as follows: male EBV-transformed human LCL from a healthy individual; female fibroblast cell line GM07693 (National Institute of General Medical Sciences, Bethesda, MD), carrying a balanced translocation, 46,X,t(X;10),(Xqter→Xp11.2::10q24.3→10qter;10pter→10q24.3::Xp11.1→Xpter) (24); normal female diploid fibroblast cell line GM01871 (National Institute of General Medical Sciences); normal female diploid fibroblast (lung) cell line WI-38 (American Type Culture Collection, Manassas, VA); LCLs from the male ICF patient PT5 (34); and fibroblast cell line from the female ICF patient PT3 (16).
All LCLs were cultured in RPMI medium 1640 supplemented with 10% FBS and 2 mM l-glutamine. The growth medium for all fibroblasts was Eagle's MEM with Earle's salt, 15% FBS, and 2 mM l-glutamine.
Three-Color and Four-Color DNA FISH.
Nuclei for 2D FISH were prepared as described in ref. 3. Cosmid probes (80 ng) encompassing the PAR2 genes (U160G10, C8.2, and LLJ136; ref. 21) and paints for the X or Y chromosomes (150 ng) were cohybridized together with 5–10 μg of human Cot1 and 5 μg of salmon sperm DNA. Paints and cosmids were labeled by nick translation with biotin-16-dUTP and digoxigenin-11-dUTP, respectively. Biotinylated probes were detected by using FITC followed by biotinylated anti-avidin and a final layer of fluorochrome-conjugated avidin. Digoxigenin-labeled probe was detected with sequential layers of rhodamine-conjugated anti-digoxigenin and Texas red-conjugated anti-sheep IgG.
In the case of four-color DNA FISH, digoxigenin-labeled probes were detected by using rhodamine anti-digoxigenin and Texas red anti-sheep IgG (Vector Laboratories, Peterborough, U.K.), biotin-labeled probes were detected by using Cy5 streptavidin and biotinylated anti-avidin (Vector Laboratories), and the X or Y chromosome paints directly labeled with FITC (Cambio, Cambridge, U.K.) were detected by using F1 rabbit anti-FITC and F2 FITC anti-rabbit (Cambio). Hybridization, washing, and detection were carried out as described in ref. 3. Slides were counterstained with 0.5 μg/ml DAPI.
Combined RNA and DNA FISH.
Cells grown on slides were fixed in 4% paraformaldehyde for 10 min, permeabilized in 0.7% Triton X-100 for 10 min, and stored in 70% ethanol. After progressive dehydration in ethanol, the cells were air-dried and hybridized overnight at 37°C in 50% formamide/2× SSC with an XIST probe (provided by C. Brown, University of British Columbia, Vancouver, BC, Canada) labeled by nick translation with digoxigenin-11-dUTP. Slides were washed twice in 50% formamide/2× SSC and once in 2× SSC. XIST signal was detected with rhodamine anti-digoxigenin and Texas red anti-sheep IgG (Vector Laboratories) with washes in 4× SSC/1% Tween 20.
After XIST visualization, cells were fixed in 4% paraformaldehyde for 30 min, denatured in 50% formamide/2× SSC at 80°C for 15 min, and rinsed in 2× SSC before overnight hybridization with biotin-16-dUTP-labeled gene-specific probe and chromosome paint directly labeled with FITC. Probe detection was as that used for DNA FISH.
Image Capture and Analysis.
Slides for 2D FISH were examined by using an Axioplan fluorescence microscope (Carl Zeiss, Welwyn, U.K.) fitted with a Chroma 84000 quadruple-band pass filter set (Chroma Technology, Rockingham, VT). Grayscale images were captured with an Orca AG CCD camera (Hamamatsu Photonics, Welwyn Garden City, Hertfordshire, U.K.). Images were collected from 50 to 100 randomly selected nuclei for each experiment and then analyzed by using a custom IPLab script that calculates the distance between the probe signals and the nearest CT edge or the distance between two signals, as described in refs. 3 and 5. Positive distances indicate that the locus is located inside of the CT, whereas negative values represent signals that are located outside of the detectable limits of the CT. The statistical significance of the data was tested by using the nonparametric Mann–Whitney and Kolmogorov–Smirnov tests of the null hypothesis. P < 0.05 has been taken as statistically significant.
For 3D analysis, the imaging system comprised a Zeiss Axiovert 200 fluorescence microscope equipped with 100×/1.4 and 63×/1.4 plan apochromat objectives, a Lambda LS 300W xenon source with liquid light guide, 10-position excitation and emission filterwheels (Sutter Instruments, Novato, CA), a PZ2000 3-axis XYZ stage with integrated piezo Z-drive (Applied Scientific Instrumentation, Eugene, OR), and a Photometrics Coolsnap HQ2 CCD camera (Roper Scientific, Tucson, AZ). The filterwheels were fitted with a #86000 Sedat quad set with single emission filters (Chroma Technology). Image capture was performed by using IPLab v4.0 for PC (BD Biosciences, Rockville, MD).
Supplementary Material
Acknowledgments
We thank P. Perry for helpful software assistance; C. Brown for providing XIST probe; and A. Cerase for help in the initial part of the work, C. Morey for help with statistical analysis, and both for stimulating discussion. This work was supported by Provincia di Napoli Grant 02 PEG 42 bil.2006 (to M.R.M.), Telethon Grant GGP03208 and a grant from Provincia di Napoli (to M.D.), the U.K. Medical Research Council, European Union Sixth Framework Programme Network of Excellence Epigenome Grant LSHG-CT-2004-503433, and the Short Mobility Program from the National Research Council (M.M.). W.A.B. is a Centennial Fellow of the James S. McDonnell Foundation.
Abbreviations
- CI
confidence interval
- CT
chromosome territory
- ICF
immunodeficiency centromeric instability facial anomalies
- LCL
lymphoblast cell line
- PAR
pseudoautosomal region
- Xa
active X chromosome
- XCI
X chromosome inactivation
- Xi
inactive X chromosome.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702924104/DC1.
References
- 1.Heard E, Bickmore W. Curr Opin Cell Biol. 2007;19(3):311–316. doi: 10.1016/j.ceb.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Mahy NL, Perry PE, Gilchrist S, Baldock RA, Bickmore WA. J Cell Biol. 2002;157:579–589. doi: 10.1083/jcb.200111071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahy NL, Perry PE, Bickmore WA. J Cell Biol. 2002;159:753–763. doi: 10.1083/jcb.200207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JM, Leach J, Reittie JE, Atzberger A, Lee-Prudhoe J, Wood WG, Higgs DR, Iborra FJ, Buckle VJ. J Cell Biol. 2006;172:177–187. doi: 10.1083/jcb.200507073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambeyron S, Bickmore WA. Genes Dev. 2004;18:1119–1130. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morey C, Da Silva NR, Perry P, Bickmore WA. Development (Cambridge, UK) 2007;134:909–919. doi: 10.1242/dev.02779. [DOI] [PubMed] [Google Scholar]
- 7.Williams RR, Broad S, Sheer D, Ragoussis J. Exp Cell Res. 2002;272:163–175. doi: 10.1006/excr.2001.5400. [DOI] [PubMed] [Google Scholar]
- 8.Volpi EV, Chevret E, Jones T, Vatcheva R, Williamson J, Beck S, Campbell RD, Goldsworthy M, Powis SH, Ragoussis J, et al. J Cell Sci. 2000;113(9):1565–1576. doi: 10.1242/jcs.113.9.1565. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert N, Boyle S, Fiegler H, Woodfine K, Carter NP, Bickmore WA. Cell. 2004;118:555–566. doi: 10.1016/j.cell.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, et al. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- 11.Moen PT, Jr, Johnson CV, Byron M, Shopland LS, de la Serna IL, Imbalzano AN, Lawrence JB. Mol Biol Cell. 2004;15:197–206. doi: 10.1091/mbc.E03-06-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shopland LS, Johnson CV, Byron M, McNeil J, Lawrence JB. J Cell Biol. 2003;162:981–990. doi: 10.1083/jcb.200303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos AP, Abranches R, Stoger E, Beven A, Viegas W, Shaw PJ. J Cell Sci. 2002;115:4597–4605. doi: 10.1242/jcs.00160. [DOI] [PubMed] [Google Scholar]
- 14.Matarazzo MR, De Bonis ML, Gregory RI, Vacca M, Hansen RS, Mercadante G, D'Urso M, Feil R, D'Esposito M. Hum Mol Genet. 2002;11:3191–3198. doi: 10.1093/hmg/11.25.3191. [DOI] [PubMed] [Google Scholar]
- 15.Bourc'his D, Miniou P, Jeanpierre M, Molina GD, Dupont J, Saint-Basile G, Maraschio P, Tiepolo L, Viegas-Péquignot E. Cytogenet Cell Genet. 1999;84:245–252. doi: 10.1159/000015269. [DOI] [PubMed] [Google Scholar]
- 16.Hansen RS, Stöger R, Wijmenga C, Stanek AM, Canfield TK, Luo P, Matarazzo MR, D'Esposito M, Feil R, Gimelli G, et al. Hum Mol Genet. 2000;9:2575–2587. doi: 10.1093/hmg/9.18.2575. [DOI] [PubMed] [Google Scholar]
- 17.Gisselsson D, Shao C, Tuck-Muller CM, Sogorovic S, Palsson E, Smeets D, Ehrlich M. Chromosoma. 2005;114:118–126. doi: 10.1007/s00412-005-0343-7. [DOI] [PubMed] [Google Scholar]
- 18.De Bonis ML, Cerase A, Matarazzo MR, Ferraro M, Strazzullo M, Hansen RS, Chiurazzi P, Neri G, D'Esposito M. Hum Mol Genet. 2006;15:1123–1132. doi: 10.1093/hmg/ddl027. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich M, Buchanan KL, Tsien F, Jiang G, Sun B, Uicker W, Weemaes CM, Smeets D, Sperling K, Belohradsky BH, et al. Hum Mol Genet. 2001;10:2917–2931. doi: 10.1093/hmg/10.25.2917. [DOI] [PubMed] [Google Scholar]
- 20.D'Esposito M, Ciccodicola A, Gianfrancesco F, Esposito T, Flagiello L, Mazzarella R, Schlessinger D, D'Urso M. Nat Genet. 1996;13:227–229. doi: 10.1038/ng0696-227. [DOI] [PubMed] [Google Scholar]
- 21.Ciccodicola A, D'Esposito M, Esposito T, Gianfrancesco F, Migliaccio C, Miano MG, Matarazzo MR, Vacca M, Franze A, Cuccurese M, et al. Hum Mol Genet. 2000;9:395–401. doi: 10.1093/hmg/9.3.395. [DOI] [PubMed] [Google Scholar]
- 22.Hepperger C, Otten S, von Hase J, Dietzel S. Chromosoma. 2007;116:117–133. doi: 10.1007/s00412-006-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeesch JR, Petit P, Kermouni A, Renauld JC, Van Den Berghe H, Marynen P. Hum Mol Genet. 1997;6:1–8. doi: 10.1093/hmg/6.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Carrel L, Willard HF. Proc Natl Acad Sci USA. 1999;96:7364–7369. doi: 10.1073/pnas.96.13.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota H, Singer MJ, van den Engh GJ, Trask BJ. Chromosome Res. 1997;5:157–166. doi: 10.1023/a:1018438729203. [DOI] [PubMed] [Google Scholar]
- 26.Matarazzo MR, De Bonis ML, Strazzullo M, Cerase A, Ferraro M, Vastarelli P, Ballestar E, Esteller M, Kudo S, D'Esposito M. J Cell Physiol. 2007;210:711–719. doi: 10.1002/jcp.20879. [DOI] [PubMed] [Google Scholar]
- 27.Blaschke RJ, Rappold G. Curr Opin Genet Dev. 2006;16:233–239. doi: 10.1016/j.gde.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Dietzel S, Schiebel K, Little G, Edelmann P, Rappold GA, Eils R, Cremer C, Cremer T. Exp Cell Res. 1999;252:363–375. doi: 10.1006/excr.1999.4635. [DOI] [PubMed] [Google Scholar]
- 29.Chaumeil J, Le Baccon P, Wutz A, Heard E. Genes Dev. 2006;20:2223–2237. doi: 10.1101/gad.380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemson CM, Hall LL, Byron M, McNeil J, Lawrence JB. Proc Natl Acad Sci USA. 2006;103:7688–7693. doi: 10.1073/pnas.0601069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gartler SM, Riggs AD. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- 32.Eils R, Dietzel S, Bertin E, Schrock E, Speicher MR, Ried T, Robert-Nicoud M, Cremer C, Cremer T. J Cell Biol. 1996;135:1427–1440. doi: 10.1083/jcb.135.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sproul D, Gilbert N, Bickmore WA. Nat Rev Genet. 2005;6:775–781. doi: 10.1038/nrg1688. [DOI] [PubMed] [Google Scholar]
- 34.Wijmenga C, Hansen RS, Gimelli G, Björck EJ, Davies EG, Valentine D, Belohradsky BH, van Dongen JJ, Smeets DF, van den Heuvel LP, et al. Hum Mutat. 2000;16:509–517. doi: 10.1002/1098-1004(200012)16:6<509::AID-HUMU8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.