Abstract
Bacteria can communicate via diffusible signal molecules they generate and release to coordinate their behavior in response to the environment. Signal molecule concentration is often proportional to bacterial population density, and when this reaches a critical concentration, reflecting a bacterial quorum, specific behaviors including virulence, symbiosis, and horizontal gene transfer are activated. Quorum-sensing regulation in many Gram-negative bacteria involves acylated homoserine lactone signals that are perceived through binding to LuxR-type, acylated-homoserine-lactone-responsive transcription factors. Bacteria of the rhizobial group employ the LuxR-type transcriptional activator TraR in quorum sensing, and its activity is further regulated through interactions with the TraM antiactivator. In this study, we have crystallographically determined the 3D structure of the TraR–TraM antiactivation complex from Rhizobium sp. strain NGR234. Unexpectedly, the antiactivator TraM binds to TraR at a site distinct from its DNA-binding motif and induces an allosteric conformational change in the protein, thereby preventing DNA binding. Structural analysis reveals a highly conserved TraR–TraM interface and suggests a mechanism for antiactivation complex formation. This structure may inform alternative strategies to control quorum-sensing-regulated microbial activity including amelioration of infectious disease and antibiotic resistance. In addition, the structural basis of antiactivation presents a regulatory interaction that provides general insights relevant to the field of transcription regulation and signal transduction.
Keywords: crystal structure, TraR–TraM complex, allosteric mechanism, protein–protein interaction, signal transduction
Bacteria can release signal molecules into their environment and subsequently respond to these same signals, as a measure of their own population density (1). Generally known as quorum sensing, this mechanism regulates processes such as virulence, symbiosis, and horizontal gene transfer, which are of adaptive benefit in dense populations and are typified by processes associated with host organisms. Bacteria within the large and diverse proteobacterial group use acylated homoserine lactones (AHLs) as quorum-sensing signal molecules (2). AHLs are usually synthesized via enzymes of the LuxI family, and the response to these signals is typically mediated through transcription factors of the LuxR family. There has been intense interest in studying the molecular mechanisms of quorum sensing to develop strategies by which to control microbial activity. Inhibition of AHL quorum sensing through chemically synthesized AHL analogs, inhibitory natural products, and AHL-degrading enzymes has achieved variable degrees of effectiveness (3). Inhibition mechanisms, however, are still required and offer the promise of ameliorating infectious disease through modulation of intercellular communication.
Proteobacteria within the Rhizobiaceae and Bradyrhizobiaceae families express homologs of the TraM protein, a potent antiactivator protein originally identified in Agrobacterium tumefaciens that blocks the activity of its associated LuxR-type transcription factor, TraR. TraM inhibits TraR in several different microbial taxa and is often required to maintain the quorum-sensing mechanism in the inactive state (4–7). TraM inhibits quorum sensing by direct binding to TraR, preventing it from binding to its DNA target promoters (8, 9). Although the structure of TraM from A. tumefaciens has been solved recently (10–12), this discovery has provided only limited insight into the mechanism of TraR inhibition. We now report the 3D structure of the TraM (TraMNGR) inhibitor from Rhizobium species strain NGR234 in complex with its cognate TraR transcription factor (TraRNGR). The novel configuration of this complex distinguishes several competing models for the inhibitory activity of TraM and suggests that heterocomplex formation allosterically and indirectly modifies the conformation of the TraR DNA binding domain, thereby blocking association with target promoters.
Results
Biochemical Characterizations.
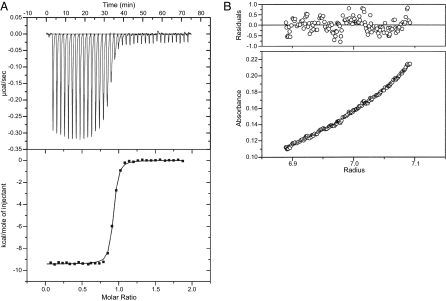
In solution, TraMNGR (theoretical molecular mass of 14.37 kDa) existed as a molecular species of 17.6 and 14.5 kDa by gel filtration and analytical ultracentrifugation (AUC) experiments [supporting information (SI) Fig. 5A], respectively, consistent with monomeric TraMNGR. Purified TraRNGR (monomer molecular mass of 26.29 kDa) eluted by gel filtration at 44.5 kDa and sedimented in AUC at 53.4 kDa (SI Fig. 5B); both values were consistent with a homodimeric structure. Isothermal titration calorimetry (ITC) suggested that TraM binds to TraR in a 1:1 molar ratio, with a disassociation constant (Kd) of 14.9 nM (Fig. 1A). The molecular mass of the TraR–TraM complex (theoretical molecular mass of 81.32 kDa), as derived from AUC, was 83.2 kDa (Fig. 1B), which was indicative of a heterotetramer (TraRNGR–TraMNGR)2.
Fig. 1.
Biochemical characterizations of the TraRNGR–TraMNGR complex. (A) ITC analysis of the TraRNGR–TraMNGR interaction. A single binding site was used to fit the data and to derive thermodynamic parameters. (B) AUC sedimentation equilibrium studies on the TraRNGR–TraMNGR complex. A single species model was used to fit data. Data fitting (Upper) and the fitting residual (Lower) are shown.
Overall Structure of TraRNGR–TraMNGR.
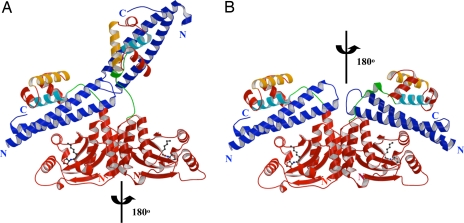
TraRNGR–TraMNGR crystallized in the P1 space group with one heterotetramer complex per asymmetric unit. TraRNGR was organized into two structural and functional domains: the N-terminal dimerization domain (NTD) (1–164 aa) and the C-terminal DNA-binding domain (CTD) (175–236 aa). In the structure, the two NTDs of the complex were related by a rotational C2 symmetry. Interestingly, CTDs were located asymmetrically relative to their respective NTDs. In one monomer (Fig. 2A), the CTD was packed over its NTD via an 11-aa linker that looped back (the closed form). In the second monomer, the CTD swung far from its NTD (the open form) and the linker adopted an extended conformation. In the closed form, TraMNGR was sandwiched between the TraRNGR NTD and CTD, interacting with both domains. The interaction with the NTD was, however, completely disrupted in the open form, and the resulting extended conformation was stabilized by contact with the neighboring TraRNGR–TraMNGR in the crystal. Without crystal packing, it is possible that both TraRNGR protomers would adopt the closed conformation, each TraMNGR interacting with the NTD and CTD of a single TraRNGR protomer. A model of (TraRNGR–TraMNGR)2 was thus generated assuming that the two TraRNGR–TraMNGR pairs follow the C2 rotational symmetry identified in NTDs, and no steric clashes were observed (Fig. 2B).
Fig. 2.
Overall structure of the TraRNGR–TraMNGR complex. (A) Structure of tetrameric NGR234 TraR–TraM complex. The TraRNGR–TraMNGR pair in the closed conformation is colored red and blue, respectively, whereas the other pair in the open conformation is in dark red and dark blue, respectively. The ligand AHL is shown in a ball-and-stick representation. α10, the major TraM-binding site, and α12, the DNA recognition helix, are colored in cyan and orange, respectively. The linker is colored in green. (B) Model of symmetric (TraRNGR–TraMNGR)2 in solution. The model was generated by applying the C2 rotational symmetry of the NTDs to the closed conformation of dimeric TraRNGR–TraMNGR. No structural conflicts are observed in the symmetric model. Views of A and B are the same. The figures were generated by using MOLSCRIPT and RASTER 3D (28, 29).
Interaction Between TraRNGR and TraMNGR.
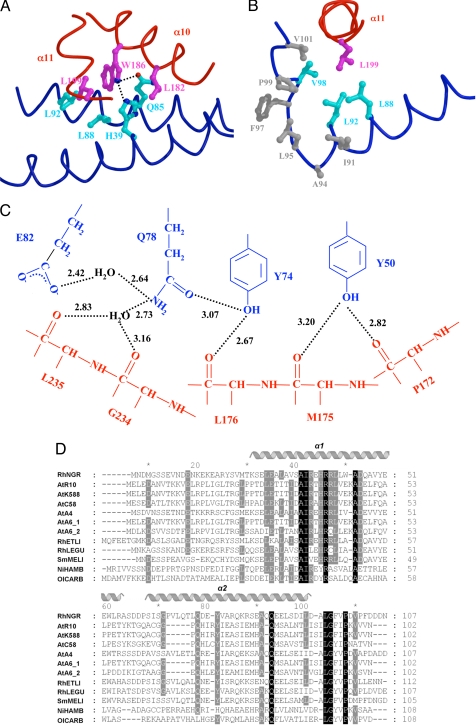
The interactions between the CTD and TraMNGR were maintained in both the open and closed conformations of TraRNGR. The TraRNGR CTD residues in α10 and α11 provided the majority of contacts with TraMNGR. L182, W186, and P178 of α10 projected their side chains into the groove formed between the two long helices of TraMNGR. Mutational analysis of the A. tumefaciens system (TraRAt–TraMAt) has revealed that modification of P178 and L182 either decreases or abolishes the antiactivation of TraRAt by TraMAt (9). In the TraRNGR–TraMNGR structure, W186, conserved among LuxR-type proteins (SI Fig. 6), was completely buried by a pocket formed by H39, Q85, L88, and L92 of TraMNGR (Fig. 3A). Besides nonspecific van der Waals interactions, atom NE1 of W186 formed intermolecular hydrogen bonds with both NE2 of H39 and OE1 of Q85 (Fig. 3A). For α11, the interaction is primarily via a hydrophobic cluster with the C-terminal end of TraMNGR, a region that has been implicated in TraR binding for TraMAt (8). L199 was located at the center of this cluster (Fig. 3B) and was also conserved (L or I) among LuxR proteins (SI Fig. 6).
Fig. 3.
Structural analysis of the TraRNGR–TraMNGR complex. (A) The TraRNGR–TraMNGR interactions at TraRNGR W186. Side chains from TraRNGR are colored in magenta, and those from TraMNGR are in cyan. The oxygen atom is in red, and nitrogen atoms are in blue. (B) Interactions of TraRNGR L199 (in magenta) with the hydrophobic cluster at the C termini of TraMNGR. Hydrophobic side chains of the cluster are colored in gray, whereas those that interact directly with L199 are colored in cyan. (C) The extensive intermolecular hydrogen-bonding network. Residues from TraMNGR and TraRNGR are colored in blue and red, respectively. The values (in angstroms) denote the distance between hydrogen donors and acceptors. Only interacting moieties, i.e., side chains of TraMNGR and backbones of TraRNGR, are shown. (D) Multiple sequence alignment of the antiactivator TraM proteins by using CLUSTALW (30). Amino acid sequences are from the following bacteria: RhNGR (or TraMNGR in the text), Rhizobium sp. NGR234; AtR10 (TraMAt), A. tumefaciens R10; AtK588, A. tumefaciens K588; ATC58, A. tumefaciens C58; AtA4, A. tumefaciens A4; AtA_1, A. tumefaciens A6; AtA_2, A. tumefaciens A6 traM2; RhETLI, Rhizobium etli CFN42; RhLEGU, R. leguminosarum; SmMELI, Sinorhizobium meliloti AK631; and NiHAMB, Nitrobacter hamburgensis X14; OlCARB, Oligotropha carboxidovorans. Invariant residues are highlighted in black, and highly conserved resides are shaded in grey. Secondary structure elements of TraMNGR are indicated above. A and B were generated by using MOLSCRIPT and RASTER 3D (28, 29).
Similarly, the TraM residues that participate in the TraR–TraM interaction were also conserved. TraMNGR H39 and Q85 interacted with TraRNGR W186 and were conserved among most TraM proteins (Fig. 3D). Several other conserved TraMNGR residues were engaged in hydrogen bond interactions with TraRNGR: (i) Y74 was centered within an extensive nine-residue intermolecular hydrogen-bonding network (Fig. 3C); (ii) R40 was hydrogen bonded to L199 and N198; and (iii) Q71 hydrogen bonded with L170. All of the hydrogen bonds involving these three conserved TraM residues (R40, Q71, and Y74) occurred between TraMNGR side chains and backbone atoms of TraRNGR, and therefore these interactions were somewhat independent of the TraR primary sequence. Although the W186 (TraR) side chain contributed to hydrogen bonding (described above), the conserved nature of this residue suggests that these hydrogen bonds are general to TraM–TraR interactions and that W186 plays a critical role in recognizing specific TraM side chains during complex formation. Taken together, these findings suggest that the extensive and complex intermolecular hydrogen bond patterns observed in NGR234 should be general to the TraR–TraM interaction in related systems. These findings provide structural information that largely corroborates the extensive mutational analyses of TraR and TraM proteins from A. tumefaciens (8, 13, 14). Some of the corresponding residues are clearly implicated in the NGR234 antiactivation complex structure, whereas others may play transient roles in complex formation or additional ancillary functions.
In the closed conformation of TraRNGR, the NTD also interacted with TraMNGR but less extensively. Notably, none of the interacting residues was conserved within either the TraM or TraR families. A much smaller surface area was sequestered within TraRNGRNTD–TraMNGR contacts (≈988 Å2) than by those of TraRNGRCTD–TraMNGR (≈2,180 Å2). The minor role of the NTD in binding of TraMNGR may account for the open conformation in the crystal, where its interactions with TraMNGR were disrupted. These structural observations were consistent with previous mutational analysis on TraRAt, which have suggested that the TraR CTD is mainly responsible for TraM interactions (9).
The flexible linker (residues 165–174), which tethered the TraR CTD and NTD, also interacted with TraMNGR, and this mode of the interaction was preserved in both the open and closed conformations. In particular, the L170 and P172 backbones of TraRNGR were hydrogen bonded to the conserved TraMNGR residues Q71 and Y50, a less well conserved position in the protein (Fig. 3D).
Structures of TraMNGR and TraRNGR.
TraMNGR folded into two long antiparallel α-helices, similar to the structure of monomeric TraMAt from A. tumefaciens (10–12). The two-helix bundles of two adjacent TraMAt molecules further interacted to promote a homodimeric structure, stabilized by the hydrophobic molecular surface of TraMAt that was largely buried along the dimer interface. Mutational studies indicate that this configuration maintains the stability of TraMAt (10). Notably, the positions of hydrophobic residues sequestered within the dimer interface of TraMAt (L14, L17, L20, I70, and I77) contained charged or polar residues in TraMNGR (N13, K16, R19, E73, and K79). TraMNGR was primarily hydrophilic on the surface and thus was able to exist as a monomer, as indicated by both gel filtration and AUC studies.
TraRNGR was similar to TraRAt (44.1% sequence homology; SI Fig. 6), the crystal structure of which, with its AHL and a DNA target sequence (the activation complex), has been solved (15, 16). As with TraRAt, the TraRNGR NTD contains the AHL-binding site including the essential D72 residue (14), with the AHL molecule embedded within a largely hydrophobic core of this domain. The helical TraRNGR CTD contains the DNA recognition helix (α12), which functions to interact with target DNA sequences through the major groove (7, 15, 16). The overall structure of the TraR NTD and CTD in the TraRNGR–TraMNGR antiactivation complex and the TraRAt–DNA complex overlayed well with rmsds of <2.5 and <1.2 Å, respectively.
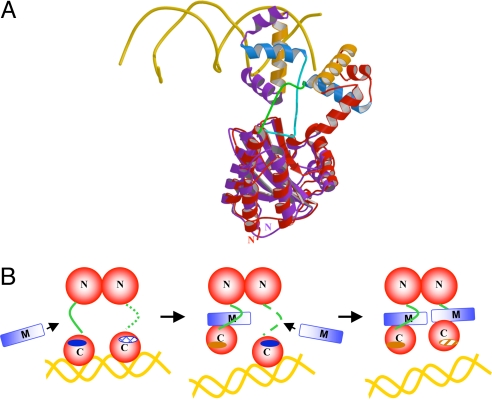
The relative orientation of the TraR CTD with respect to its NTD varied greatly between the A. tumefaciens and NGR234 complexes and also between protomers within the same complex. In TraRNGR–TraMNGR, one of the TraRNGR CTDs along with the bound TraMNGR was articulated to make nonspecific contacts with the neighboring molecule because of crystal packing. In the TraRAt–DNA complex, the two CTDs shift independently to present α12 in an optimal distance and orientation to make contact with the DNA (15, 16). When the entire A. tumefaciens and NGR234 complexes were superimposed on the NTD, the CTD orientation varied drastically (Fig. 4A). These findings suggest that each CTD can move as a discrete unit, independent from the NTD, that may adopt various orientations owing to the flexible linker between the CTD and NTD. Fig. 4A shows that this linker also underwent a remarkable structural shift upon TraM binding. In the TraRAt–DNA activation complex, the linker is exposed on the surface of the complex (15, 16). In the TraRNGR–TraMNGR complex, the linker was found to be rotated 180° around I163 and to be sequestered along the dimeric interface formed by the TraR NTDs. Consequently, the DNA recognition helix (α12) was found to be rotated ≈90°, well out of position to interact with the DNA major groove.
Fig. 4.
Mechanism of TraM inhibition of TraR. (A) Comparison of TraRAt–DNA with TraRNGR–TraMNGR structures. The NTDs of the both structures are superimposed, but for clarity, only one protomer of TraRNGR (in red) and TraRAt (in purple) from each structure is shown. α10, the TraR helix primarily responsible for TraM interactions, is light blue, and α12, the DNA binding helix, is orange. DNA is displayed as a double coil and is gold. The linker of TraRNGR is highlighted in green, and that of TraRAt is in cyan. The image was generated by using MOLSCRIPT and RASTER 3D (28, 29). (B) The proposed stepwise dissociation of TraRNGR–DNA by TraMNGR. One of the linkers in TraR–DNA, disordered and thus not observed crystallographically, is represented by the dotted line. The exposed TraM-binding site is indicated by a dark blue solid oval, and the buried site is denoted by a hatch-filled oval. The DNA-binding site facing the reader is represented as a dark orange solid oval, and that facing away from the reader as a shaded oval.
Discussion
It is clear that TraM can associate with TraR that is free in solution, as well as with TraR that is preassociated at a DNA-binding site (8, 10, 11, 13). For free TraR, our findings with the NGR234 complex suggest a relatively simple mechanism. TraMNGR monomers bind independently to each TraR protomer in the TraRNGR dimer. It may be that binding of the first TraMNGR promotes binding of the second TraMNGR because the tetrameric TraM–TraR NGR234 complex was the dominant species observed in vitro. Consistent with this, the conformational changes induced in one protomer of TraRNGR upon TraM binding would likely make α10 in the adjacent protomer more accessible.
A more complex mechanism must be considered for interactions between TraM and TraR that is already bound to DNA. The NGR234 TraR–TraM complex revealed the positions that provide important contacts with TraR. The corresponding positions on TraR, encompassed by the linker and α10, were readily accessible in the TraRAt–DNA complex (Fig. 4A). A mechanism was suggested by which TraM might disengage TraR that is already associated with its DNA-binding site (Fig. 4B). We describe this model in terms of the NGR234 tetrameric complex, but similar mechanisms could also lead to the octameric A. tumefaciens complex from a preformed TraR–DNA complex. Briefly, one TraM may bind to the exposed linker and α10 of one TraR protomer in the TraR2–DNA complex. Binding of TraM drives the linker to rotate inward and repositions α12, thereby disengaging this TraR protomer from its half-site on the DNA. In the TraRAt–DNA structure, α10 of the other TraR protomer is buried within the structure. Upon the dissociation of the first TraR protomer from the DNA by TraM, the buried α10 would be exposed, allowing it to interact with a second TraM that may already have associated with the TraR linker region. Such a stepwise dissociation, coupled with the strong affinity of TraM to TraR (Kd of 14.9 nM; described above), would promote disruption of cooperativity and hence destabilize the binding of homodimeric TraR to DNA. This mechanism suggests that a transient ternary complex (TraM–TraR2–DNA) may form, although this may be very short lived. This ternary intermediate has been detected in A. tumefaciens when incubating TraMAt with the TraRAt–DNA complex (13).
Transcription factors often bind specific sequences associated with target genes. Antiactivators from several systems appear to occupy sites on the transcription factors that would otherwise coordinate specific base contacts on the DNA, thereby precluding or inhibiting binding of the transcription factor to its target elements (17–19). A different mechanism is for the antiactivator to occlude sequences required for requisite multimerization of transcription factors into their active form (20, 21). The TraR–TraM complex structure we report here provides a different mechanism by which the antiactivator allosterically prevents DNA binding by indirectly altering the conformation of the DNA binding domain, preventing productive interactions with DNA-binding sites. This allosteric mechanism of inhibition may be more broadly used by antiactivators than is currently appreciated for transcription regulation and complex signal transduction pathways.
Materials and Methods
Protein Expression and Purification.
The traM and traR genes from Rhizhobium sp. strain NGR234 were cloned into pET11a (Novagen, Madison, WI) and pET23b (Novagen) overexpression vectors, and the plasmids were transformed into Escherichia coli BL21(DE3) codon plus and Rosetta 2, respectively. To overexpress TraMNGR, cells were grown in LB to an OD600 of 0.8 at 37°C and induced for 5 h with isopropyl-β-d-thiogalactopyranoside (0.4 mM). To overexpress TraRNGR, after cells were grown in LB at 37°C to an OD600 of 0.6, N-(3-oxo-octanoyl)-l-homoserine lactone (25 μM) and isopropyl-β-d-thiogalactopyranoside (0.1 mM) were added, and the culture was grown 5 h at 25°C.
To purify TraMNGR, cells were lysed in 50 mM sodium phosphate (pH 8.0)/0.5 mM EDTA/1 mM DTT/50 mM NaCl by using a continuous flow microfluidizer (MicroFluidics, Taylorsville, UT). Clear cell lysate was loaded on a FastQ column (Amersham Biosciences, Piscataway, NJ) and eluted with a gradient of NaCl (0.05–1 M). Fractions containing TraMNGR were concentrated, exchanged to 50 mM Tris·Cl (pH 8.0)/200 mM NaCl/0.5 mM EDTA/1 mM DTT and purified by using a Superdex75 column (Amersham Biosciences). For TraRNGR purification, cells were lysed in 50 mM imidazole (pH 8.0)/0.5 mM EDTA/300 mM NaCl/1 mM DTT/5% glycerol. Cell-free lysate was loaded onto a Heparin column (Amersham Biosciences) and eluted with a NaCl gradient (0.30–1 M). Fractions containing TraRNGR were concentrated, exchanged to 50 mM imidazole (pH 8.0)/300 mM NaCl/0.5 mM EDTA/1 mM DTT, and size fractionated on a Superdex75 column. To prepare the TraMNGR–TraRNGR complex, purified TraRNGR and TraMNGR were mixed at a molar ratio of 1:2 and incubated at 4°C overnight in 50 mM imidazole (pH 8.0)/1 M NaCl/0.5 mM EDTA/1 mM DTT. The solution was concentrated and size fractionated on a Superdex200 column (Amersham Biosciences).
AUC Experiments.
Sedimentation equilibrium experiments were carried out by using an XL-A analytical ultracentrifuge (Beckman, Fullerton, CA). Protein samples were dialyzed extensively against a buffer containing 50 mM Hepes (pH 8.0), 300 mM NaCl, 1 mM β-methylphenylalanine, 0.5 mM EDTA, and 5% glycerol. For each protein, absorbances at 280, 275, and 285 nm were measured for three protein concentrations (0.15 mg/ml, 0.3 mg/ml, and 0.5 mg/ml) and different rotor speeds (TraMNGR, 35,000 rpm; TraRNGR, 26,000 rpm; TraMNGR–TraRNGR, 20,000 rpm) at 4°C. Sedimentation equilibrium profiles were analyzed by using the Windows (Microsoft, Redmond, WA) version of Ultrascan 8.0 (University of Texas Health Science Center, San Antonio, TX).
ITC Experiments.
ITC experiments were carried out at 25°C in a VP-ITC titration calorimeter system (MicroCal, Northampton, MA). Purified TraRNGR (120 μM) and TraMNGR (11.4 μM) were dialyzed in 50 mM imidazole (pH 7.0), 300 mM NaCl, 0.5 mM EDTA, and 1 mM β-mercaptoethanol. Thirty aliquots of 10-μl samples of TraRNGR were injected into the TraMNGR solution at 240-s intervals. Data were processed with the Origin software (OriginLab, Northampton, MA), and thermodynamic parameters of the binding process were derived by fitting the corrected binding isotherm to a single-site binding model.
Crystallization and Data Collection.
The TraMNGR–TraRNGR complex was crystallized by hanging-drop vapor diffusion by mixing an equal volume of the complex [8 mg/ml in 50 mM imidazole (pH 7.0)/300 mM NaCl/0.5 mM EDTA/1 mM DTT] with the well solution [1 mM DTT with 60 mM Hepes (pH 7.5),/120 mM CaCl2/16.8% PEG400]. Crystals were flash frozen in the well solution with a 10% increment of every ingredient plus 17% polyethylene glycol and 15% glycerol. Data were collected at ALS Beamline 4.2.2 (Advanced Light Source, Berkeley, CA) and processed by using d*trek software (22). The crystal belonged to the P1 space group (a = 56.91 Å, b = 62.50 Å, c = 65.50 Å, α = 94.89°, β = 110.47°, and γ = 99.18°) with one heterotetramer TraRNGR–TraMNGR complex per asymmetric unit.
Structure Determination.
The structure was solved by molecular replacement by using Phaser (23). Briefly, the two NTDs of TraRNGR were located by using the NTD of TraRAt (Protein Data Bank ID code 1L3L) as the initial model, and after refinement of the partial model by using Crystallography & NMR System software (24), one CTD was subsequently identified. The second CTD was found after refinement and rebuilding of the more complete model. Further refinement and noncrystallographic symmetry averaging with the use of MAMA (25) gave rise to electron density for two TraMNGR molecules, the model for which was subsequently built by using ARP/wARP (26). Further model building was performed manually in O (27), and the model was refined by using Crystallography & NMR System software (24). Crystallographic statistics are summarized in SI Table 1.
Supplementary Material
Acknowledgments
We thank Robert Schleif for critical input, Jay Nix for assistance in the data collection at ALS Beamline 4.2.2 (Advanced Light Source, Berkeley, CA), and Xuesong He (Indiana University) for providing plasmids of TraRNGR and TraMNGR. This work was supported by National Science Foundation Grant MCB-0416447 and National Institutes of Health Grant GM065260-01 (to L.C.).
Abbreviations
- AHL
acylated homoserine lactone
- AUC
analytical ultracentrifugation
- CTD
C-terminal DNA-binding domain
- ITC
isothermal titration calorimetry
- NTD
N-terminal dimerization domain.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2Q0O).
This article contains supporting information online at www.pnas.org/cgi/content/full/0704843104/DC1.
References
- 1.Waters CM, Bassler BL. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua C, Greenberg EP. Nature Rev. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 3.Zhang LH, Dong YH. Mol Microbiol. 2004;53:1563–1571. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Zhang HB, Chen G, Chen L, Zhang LH. J Bacteriol. 2006;188:2435–2445. doi: 10.1128/JB.188.7.2435-2445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuqua C, Burbea M, Winans SC. J Bacteriol. 1995;177:1367–1373. doi: 10.1128/jb.177.5.1367-1373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang I, Cook DM, Farrand SK. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Chang W, Pierce DL, Seib LO, Wagner J, Fuqua C. J Bacteriol. 2003;185:809–822. doi: 10.1128/JB.185.3.809-822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swiderska A, Berndtson AK, Cha MR, Li L, Beaudoin GM, III, Zhu J, Fuqua C. J Biol Chem. 2001;276:49449–49458. doi: 10.1074/jbc.M107881200. [DOI] [PubMed] [Google Scholar]
- 9.Luo ZQ, Qin Y, Farrand SK. J Biol Chem. 2000;275:7713–7722. doi: 10.1074/jbc.275.11.7713. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Malenkos JW, Cha MR, Fuqua C, Chen L. Mol Microbiol. 2004;52:1641–1651. doi: 10.1111/j.1365-2958.2004.04110.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Wang C, Fuqua C, Zhang LH, Chen L. J Bacteriol. 2006;188:8244–8251. doi: 10.1128/JB.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vannini A, Volpari C, Di Marco S. J Biol Chem. 2004;279:24291–24296. doi: 10.1074/jbc.M401855200. [DOI] [PubMed] [Google Scholar]
- 13.Qin Y, Su S, Farrand SK. J Biol Chem. 2007;282:19979–19991. doi: 10.1074/jbc.M703332200. [DOI] [PubMed] [Google Scholar]
- 14.Luo ZQ, Smyth AJ, Gao P, Qin Y, Farrand SK. J Biol Chem. 2003;278:13173–13182. doi: 10.1074/jbc.M210035200. [DOI] [PubMed] [Google Scholar]
- 15.Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, De Francesco R, Neddermann P, Marco SD. EMBO J. 2002;21:4393–4401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang RG, Pappas T, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 17.Navarro-Aviles G, Jimenez MA, Perez-Marin MC, Gonzalez C, Rico M, Murillo FJ, Elias-Arnanz M, Padmanabhan S. Mol Microbiol. 2007;63:980–994. doi: 10.1111/j.1365-2958.2006.05567.x. [DOI] [PubMed] [Google Scholar]
- 18.Mol CD, Arvai AS, Sanderson RJ, Slupphaug G, Kavli B, Krokan HE, Mosbaugh DW, Tainer JA. Cell. 1995;82:701–708. doi: 10.1016/0092-8674(95)90467-0. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Ishima R, Tong KI, Bagby S, Kokubo T, Muhandiram DR, Kay LE, Nakatani Y, Ikura M. Cell. 1998;94:573–583. doi: 10.1016/s0092-8674(00)81599-8. [DOI] [PubMed] [Google Scholar]
- 20.Chai Y, Zhu J, Winans SC. Mol Microbiol. 2001;40:414–421. doi: 10.1046/j.1365-2958.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- 21.Masuda S, Bauer CE. Cell. 2002;110:613–623. doi: 10.1016/s0092-8674(02)00876-0. [DOI] [PubMed] [Google Scholar]
- 22.Pflugrath JW. Acta Crystallogr D Biol Crystallogr. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 23.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Acta Crystallogr D Biol Crystallogr. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 24.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 25.Kleywegt GJ. MAMA. Uppsala: Uppsala Software Factory; 1994–2007. Version 070626. [Google Scholar]
- 26.Morris RJ, Perrakis A, Lamzin VS. Methods Enzymol. 2003;374:229–244. doi: 10.1016/S0076-6879(03)74011-7. [DOI] [PubMed] [Google Scholar]
- 27.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 28.Kraulis P. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 29.Merritt EA, Bacon DJ. Methods Enzymol. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- 30.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.