Abstract
Active catheter imaging was investigated using real-time undersampled projection reconstruction (PR) combined with the temporal filtering technique of reduced field of view (rFOV). Real-time rFOV processing was interactively enabled during highly undersampled catheter imaging, resulting in improved artifact suppression with better temporal resolution than that obtained by view-sharing. Imaging with 64 to 32 projections provided a resolution of 2 × 2 × 8 mm, and four to eight true frames per second. Image artifacts were reduced when rFOV processing was applied to the undersampled images. A comparison with Cartesian rFOV showed that PR image quality is less susceptible to aliasing that results from rFOV imaging with a wholly dynamic outer FOV. Simulations and MRI experiments demonstrated that PR rFOV provides significant artifact suppression, even for a fully dynamic FOV. The near doubling of temporal resolution that is possible with PR rFOV permits accurate monitoring of highly dynamic events, such as catheter movements, and arrhythmias, such as ventricular ectopy.
Keywords: UNFOLD, reduced field of view (rFOV), projection reconstruction, interventional MRI, radial imaging, catheter tracking, temporal filtering
Undersampled projection reconstruction (PR), or radial imaging, has been employed for interventional MR guidance (1-4). PR is an acquisition method in which Np projections are acquired, each of which consists of Nr readout samples along radially arranged lines traversing the center of k-space. The temporal resolution has been limited by the undersampling artifact that arises as the number of projections is reduced. The purpose of this investigation was to improve the temporal resolution or reduce the artifacts of highly undersampled PR using reduced field of view (rFOV) imaging. PR rFOV acquires raw data undersampled by an additional factor of 2 relative to an already potentially undersampled acquisition, acquiring interleaved projection sets in alternate frames. rFOV imaging has been previously investigated with the use of PR (5-8), modeled on Cartesian UNaliasing by Fourier-encoding the overlaps using the temporal dimension (UNFOLD) (9) and rFOV methods (10,11), and applied to real-time imaging. This investigation presents the first application of combined PR and rFOV for interventional MRI. Our hypothesis was that PR rFOV would provide significant artifact suppression even for the fully dynamic FOVs encountered in interventional MRI.
Because interventions employ continuous scanning, they are well suited for rFOV techniques, which use multiple time frames to reduce aliasing. However, there are some obstacles to the application of rFOV techniques to real-time interventions. The requirement of visualizing the most recent frame in real time was recently incorporated into rFOV techniques (12). Also, in interventional MRI, the entire FOV is often highly dynamic due to breathing, catheter motion, and scan-plane changes. However, the assumption of rFOV is that the aliased part is a slowly varying function of time (9). If the aliasing has a highly dynamic component, the aliasing cannot be fully removed by rFOV processing; therefore, aliasing of these high-temporal-frequency signals will occur.
The reduction in aliasing artifact provided by Cartesian UNFOLD was described previously (9,13), and a similar description applies to PR. When the number of projections collected is reduced by a factor of 2 (see Fig. 1a), the maximum azimuthal sampling distance, defined as (Δkϕmax(Np) = Δkr · (Nr/2) · π/Np), is increased by 2. This provides a reduced FOV (compare the point spread functions (PSFs) for 64 Np vs. 32 Np in Fig. 1b and c). The radial sampling of k-space (Δkr) is not changed and is prescribed according to the object FOV (Δkr = 1/(FOV). The rFOV sampling pattern alternates between two interleaved (“even” and “odd”) projection sets (gray and black rays in Fig. 1a). This results in an aliasing pattern that rotates in alternate frames and thus oscillates in time at the Nyquist frequency (the highest temporal frequency). This frequency, here denoted ktmax, is determined by the time needed to acquire a single frame, ΔT, ktmax = 1/(2ΔT). Therefore, the temporal frequency spectrum of the aliasing is shifted in temporal frequency space and centered at the Nyquist frequency. The aliasing can be removed by filtering out high temporal frequencies using a low-pass filter with a passband equal to the fraction R (e.g., R = 0.95 for a 95% passband filter), which suppresses signals of frequencies in a window around the Nyquist frequency (ktmax). However, only the aliasing from the static (very slow-changing) component of the object (with temporal frequencies in the range of (|kt| <[1-R] · ktmax)) oscillates at (or close to) the Nyquist frequency, and only that aliasing is suppressed by the temporal filter. After rFOV processing, the low temporal frequencies are represented by a PR PSF of 2Np projections (see Fig. 1b). More dynamic objects (with temporal frequencies in the range of [1-R] · ktmax < |kt| <ktmax) produce artifacts in the passband of the filter, which are not suppressed. After rFOV processing, they are still represented with a PSF of Np projections (see Fig. 1c). Note the improved PSF (larger region without streak aliasing) of the lower spatial frequencies. This is analogous to what happens in Cartesian rFOV, although the aliasing PSF is different.
FIG. 1.
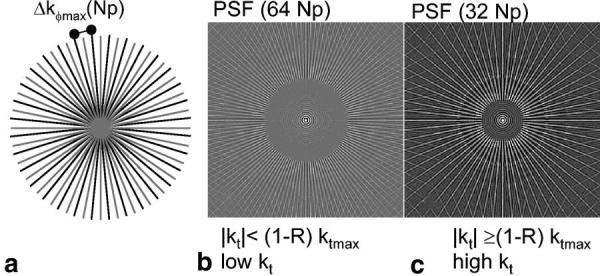
rFOV PR. a: Angularly interleaved projection sets (Np = 32) collected in alternate time frames for rFOV PR. The gray and black rays indicate separate interleaves. By reducing the number of projections collected by a factor of 2, Δkϕmax is increased and hence the FOV is reduced. After temporal filtering, the low temporal frequencies have the artifact pattern associated with a PSF of 2 · Np projections (b), while high temporal frequencies have artifacts associated with a PSF of Np (c). R is the filter passband width as a fraction of ktmax, the highest temporal frequency.
It was hypothesized in this investigation that PR rFOV image quality is less susceptible to dynamic objects in the outer FOV than Cartesian rFOV because of the former's favorable aliasing properties (14). Any aliasing from a dynamic object that is not reduced with rFOV is distributed as incoherent streaks of low signal intensity rather than as discrete ghosts (as in Cartesian imaging). Specifically, for PR rFOV, spatial frequencies below the critical frequency kr-crit(Np) = Δkr · Np · (2/π) are not undersampled, and therefore they do not contribute any aliasing (15) (Np is the number of projections in one interleaf, and Δkr is defined above). This reduces the aliasing considerably compared to Cartesian implementations. Previous studies have investigated variable-density Cartesian implementations of rFOV whereby the central k-space region is fully sampled, which reduces aliasing (10). For PR, this fully sampled central k-space is a natural consequence of its radial trajectory. The purpose of this investigation was to determine whether the use of rFOV in combination with PR improves interventional image quality through relative immunity to artifacts resulting from a fully dynamic FOV.
MATERIALS AND METHODS
Simulations
The simulations were performed with the use of Matlab (Mathworks Inc., Natick, MA). A small feature in a phantom image was used for simulation. The object was a resolution comb, gleaned from an MR phantom image, acquired with very high SNR (roughly 167:1) (Fig. 2a). A time series of images was constructed with either a static or a moving (translating) object. This object was chosen because of its distinct PR artifact pattern. The original object had no aliasing artifacts, but it was then sampled with 32 projections, acquired alternately as even or odd interleaves (as shown in Fig. 1a) in each sequential time frame. The translation of the moving object was simulated as: y(t) = yo + A · cos(2 · π · t · ΔT/T) pixels; x(t) = xo, where t = (0, 1, 2, 3, …), where A is the amplitude of the motion in pixels, and T is the period of motion. No intraframe motion was simulated. The frame time for acquiring a single image, ΔT, was set to 0.125 s. The amplitude A was varied to include 1, 2, 4, and 6 pixel amplitudes. The period of motion T was varied to include 0.5, 1.0, 2.0, 4.0, 8.0, and 16.0 s. To investigate the influence of random motion on the results of rFOV processing, noise was added to the position y(t) for the case of A = 6 pixels, by the addition of the term N(t). N(t) is normally distributed random noise with the mean and standard deviation (SD) of 0 and 1 pixel, respectively. Periodicities of T = 1.0 s represent typical cardiac motion, and periodicities of 4.0 s represent typical breathing motion periodicity. The rFOV processing was performed with a RECT95 temporal filter (defined below). The rFOV processed images were compared to 32 Np and 64 Np images of the same time frames. To assess the improvement provided by rFOV processing under conditions of motion at each amplitude and frequency, the residual undersampling artifact signal averaged over a cycle of harmonic motion was measured and normalized to the undersampling artifact in the 32 Np image.
FIG. 2.
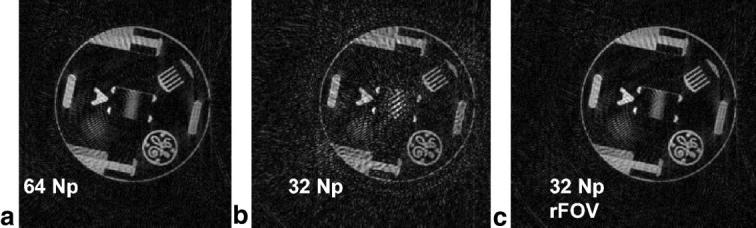
A static quality-assurance phantom reconstructed with (a) 64 projections, (b) 32 projections with no rFOV processing, and (c) 32 projections with rFOV processing with a RECT95% filter. Note the dramatic impact of artifact suppression with the use of rFOV.
Acquisition
Active catheters (Boston Scientific, Plymouth, MN) were imaged in the aorta and left ventricular cavity of five pigs, by means of a previously described interventional system (2). A real-time interactive PR balanced steady-state free precession (SSFP) sequence was implemented on a GE CV/i 1.5 T system (2). Typical scan parameters were: Nr = 160, Np = 64–32, FOV = 24–32 cm, slice thickness = 8 mm, flip angle = 60°, receiver bandwidth = ±62.5 kHz, and TR = 4.1 ms. Figure 1a shows the angularly interleaved projection sets spanning 180°, which are acquired in alternate interleaves for rFOV imaging.
Reconstruction
The real-time image reconstruction rate must match or exceed the data acquisition rate, because temporal filtering is possible only if each sequential time frame is reconstructed. In this investigation, a multi-threaded C code reconstruction was performed on a four-CPU computer (Onyx 2; Silicon Graphics) with the use of look-up table gridding (16) for speed, followed by a fast Fourier transform (www.fftw.org), a receiver coil combination using sum of squares, and a display of RGB images. Alternatively, as previously described (17-19), the signal received by the interventional devices (i.e., the catheter coils) could be displayed in only one color (red, green, or blue), while the signal from the surface coils was displayed in grayscale.
The rFOV method was performed by filtering gridded k-space in the time domain through the application of weightings to a series of the most recent raw time frames. Three temporal filters were used in this investigation. Two were low-latency filters that were designed for interventional situations in which it is essential that the most recent image be displayed in real time. One filter was very similar to that proposed previously (12) for Cartesian real-time UNFOLD with an 80% passband (here called low latency with 80% passband (LL80%)), and one was a simple modification providing a 95% passband (LL95%). The filters were designed with Matlab. They are recursive filters that use most recent raw and filtered frames as inputs (impulse responses are shown in Fig. 3). The output of such filters is (20):
| [1] |
where y(n) and x(n) are the most recent filtered and raw image frames, respectively, and y(n−1) and x(n−1) are the second-most recent filtered and raw image frames, etc. N and M are determined by the number of recursive (N) and nonrecursive (M) taps. The coefficients ak and bk are (LL80%): b = [0.4871, 1.4484, 1.4484, 0.4871] (nonrecursive taps); a = [1.0000, 1.6390, 1.0663, 0.1657] (recursive taps) and (LL95%): b = [0.7245, 2.8950, 4.3410, 2.8950, 0.7245] (nonrecursive taps); a = [1.0000, 3.5690, 4.8230, 2.9260, 0.6730] (recursive taps). The third filter is a temporal domain representation of a windowed rectangular UNFOLD filter in temporal frequency space, with 95% passband (here called RECT95%). The filter coefficients are: b = [−0.0169, 0.0852, −0.1430, 0.1825, 0.8036, 0.1825, −0.1430, 0.0852, −0.0169] (nonrecursive taps), with no recursive taps. This filter has a latency of about four time frames. Alternatively view-sharing reconstruction (adding interleaves, equivalent to a two-point mean filter) as suggested by others (21) or an unfiltered (single raw frame) reconstruction were possible. Interactive requests could be made for view-sharing, rFOV processing, or unfiltered imaging in real time.
FIG. 3.
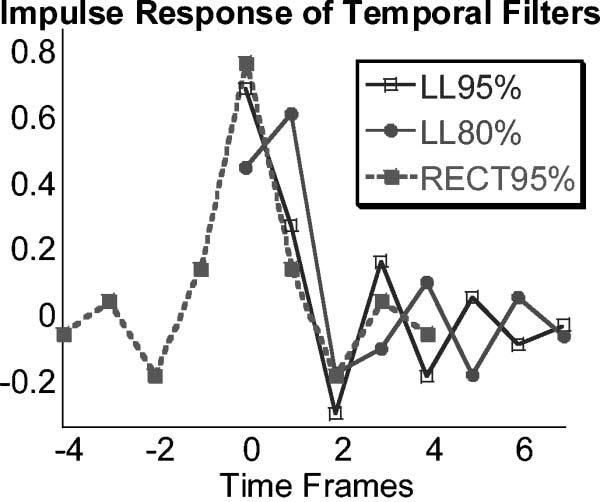
Three temporal filters were employed: two low-latency filters (LL80% and LL95%) with 80% and 95% passbands, and one that was a temporal domain representation of an UNFOLD filter with a 95% passband (RECT95%), with a latency of about four frames. The impulse responses are shown.
For a static phantom scanned in the head coil, the scan parameters were: SSFP, FOV = 18 cm, slice thickness = 8 mm, Np = 160 × 32, receiver bandwidth = ±62.5 kHz, TR/TE/θ = 4.2 ms/1.5 ms/60°. Catheter tracking and imaging was performed in anesthetized pigs, as described previously (22), and a comparison of PR rFOV and Cartesian rFOV (23) was performed with identical temporal filters. The performance of PR rFOV was visually evaluated for a reduction in artifacts. All protocols were approved by our institutional animal care and use committee.
Movie loops of real-time catheter imaging in five animal studies, with and without rFOV processing, were assessed by an MR interventionalist on a four-point scale: 1 = no significant artifacts; 2 = artifacts, but not in the region of importance; 3 = artifacts, but images are interpretable; and 4 = artifacts resulting in images that not interpretable. The interventionalist was blinded to the reconstruction method. This provided some measure of the improvement in quality gained with the rFOV method.
RESULTS
Using our custom reconstruction software, the maximum reconstruction speed achievable (independent of acquisition rate) was 10.5 frames per second (95 ms), for 160 Nr × 32 Np (four coils). Reduced FOV processing reduced the rate to 8.0 frames per second (125 ms), which increased reconstruction time by 30 ms per frame. While better computer hardware and more efficient coding would provide higher reconstruction rates, this rate was sufficient to permit real-time, online rFOV processing for Np ≥ 32.
Phantom and Simulation Results
Figure 2 shows the results of imaging a static phantom with 32 Np, using rFOV processing. This is compared to an image with 64 Np, and a 32 Np image without rFOV processing.
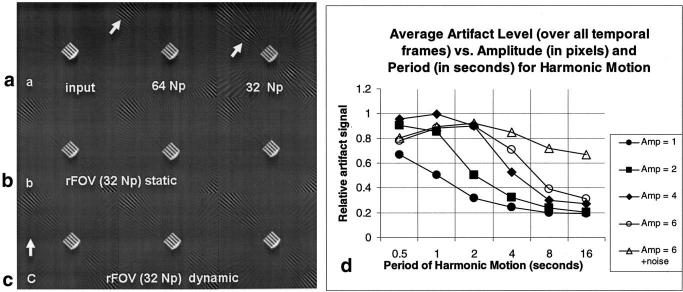
The results of the simulation are presented in Fig. 4. The top row (a) shows the original object, and the object reconstructed with 32 and 64 projections. Note the characteristic aliasing pattern at 32 and 64 Np for this object (arrows point to artifact). Figure 4b presents the results of rFOV processing for the stationary object. The artifact pattern of object is similar to that provided by 64 Np at three consecutive time frames. Figure 4c presents three consecutive time frames (ΔT = 0.125 s) of the object in motion, after rFOV processing (T = 2 s, A = 2 pixels). The artifact pattern of the moving object is similar to (but somewhat less in signal than) a 32 Np image without rFOV processing; some temporal blur is also evident. Figure 4d shows a summary of the average artifact level (averaged over all frames) after rFOV processing for many amplitudes and periods of motion. The impact of the addition of noise is also shown. The values are given as a fraction of the artifact signal before rFOV processing. When noise is added to the position, there is significant artifact even at long periods of oscillation, since the noise term introduces rapid changes in position.
FIG. 4.
Simulation of rFOV PR for a stationary and a moving object. a: Input image and image reconstructed with 64 and 32 Np. Note the characteristic artifact patterns (arrows point to artifacts). b: Stationary object at three consecutive frames after rFOV processing. c: Moving object at three consecutive frames after rFOV processing, for the case of motion with a periodicity of 2 s, and amplitude of motion of 2 pixels. The arrow indicates the direction of translation. d: Summarized results of the simulation for harmonic motion with varying periodicity and amplitude of motion, and the addition of noise in the motion. The general trend shows less artifact signal (normalized by the signal of the artifact before rFOV processing) for smaller amplitudes and longer periods of oscillatory motion. The addition of a noise term to the position results in much less artifact suppression. A periodicity of 1 s corresponds to typical cardiac motion, and a periodicity of 4 s corresponds to typical respiratory motion.
Interventional Results
Figure 5 compares the same short-axis cardiac image of a pig from a free-breathing, real-time acquisition (Np = 40) reconstructed singly (Fig. 5a), using view-sharing (Fig. 5b), with the RECT95% filter (Fig. 5c), the LL80% filter (Fig. 5d), and the LL95% filter (Fig. 5e). Signal profiles (Fig. 5f and g) across the moving septal wall show less temporal blur with rFOV than with view-sharing. Error bars represent the measured SD of the background noise. They show that the profiles across the endocardial border are significantly different with view-sharing and rFOV. The temporal blurring demonstrated in Fig. 5 appeared predictably in cardiac frames during mid-systole in each cardiac cycle during this acquisition. The LL95% filter improved the temporal resolution (less temporal blur) compared to the LL80% filter. An increase in acquisition temporal resolution would provide similar benefits.
FIG. 5.
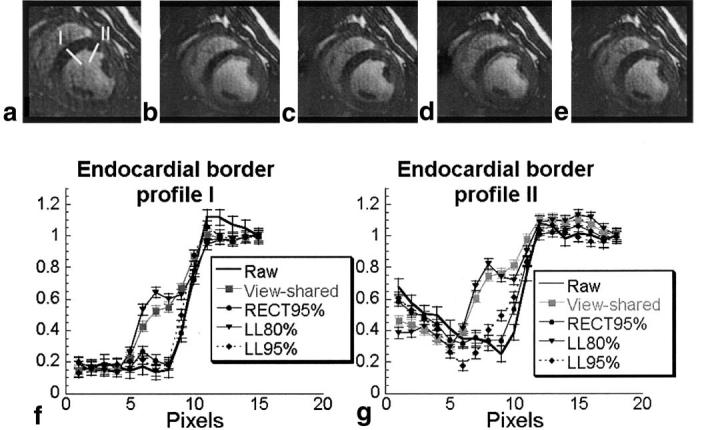
Comparison of the same 40 Np image (a) raw, and with (b) view-sharing, (c) RECT95% filter, (d) LL80% filter, and (e) LL95% filter. Two signal intensity profiles (labeled I and II), as shown in image a, were obtained crossing the endocardial border, and are plotted in f and g, respectively. The images and profiles demonstrate temporal blur of the endocardial wall, which varies with the temporal filtering applied. They show that rFOV imaging with a low-latency filter provides reduced temporal blur compared toview-sharing, but has slightly increased temporal blur compared to the RECT95% filter. The error bars on the signal intensity profiles are equal in magnitude to the noise measured in the images, and demonstrate that the differences between profiles are due to the temporal filtering applied, and not to image noise.
Figure 6 shows a 32 Np image of a catheter (colored red) within the left ventricular cavity of a pig, reconstructed without rFOV processing, and the same image reconstructed with rFOV (LL95%). The reduction in artifact is evident with rFOV.
FIG. 6.
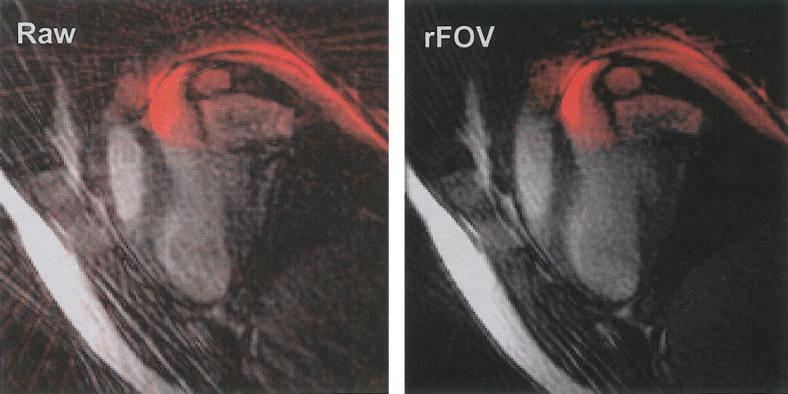
Comparison of the same 32 Np catheter image, unfiltered (left) and rFOV filtered (right). The rFOV image has better artifact suppression compared to the raw image.
Figure 7 compares Cartesian (a) and PR (b) rFOV imaging of a catheter (colored green) in the left ventricle of a pig, with matched scan parameters and slice plane. For Cartesian images, the frequency-encoding direction (indicated in Fig. 7a) was not optimal for reducing aliasing, but was optimal for visualizing the myocardial motion. Representative images from a time series show a frame with the least and the greatest amount of aliasing (labeled “least” and “most”), displayed using reconstructions with the two low-latency filters (labeled LL80% and LL95%). For both sets of images, the respiratory motion created aliasing unsuppressed by rFOV methods in some of the frames. Also for both sets of images, the use of a filter with a wider passband (LL95% as compared to LL80%) resulted in an increase in artifact in the images. However, in each case, the increase in aliasing artifact with PR rFOV (compared to Cartesian rFOV) was less obstructive to visualization of the important relevant anatomy.
FIG. 7.
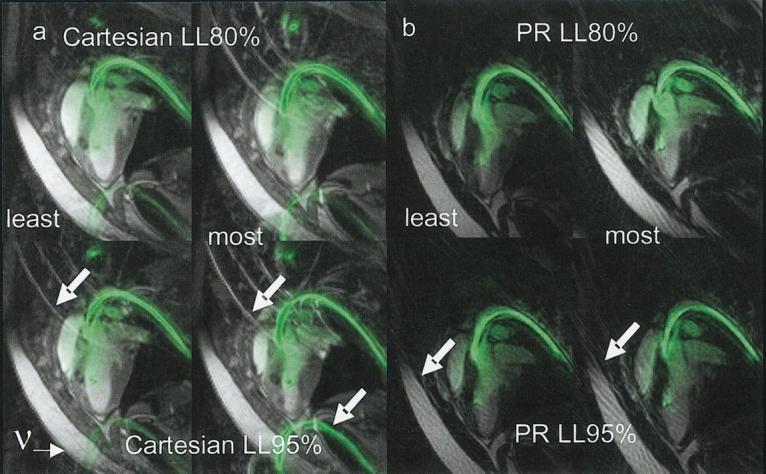
PR rFOV image with 48 Np (as compared to Cartesian rFOV with 48 phase-encodings) and matched scan parameters. Scan parameters: SSFP, TR = 4.1 ms, flip angle = 60°, receiver bandwidth = ±62.5 kHz, slice thickness = 8 mm slice. PR: FOV = 32 cm, 160 × 48 Np, Cart: FOV = 34 × 34 cm, 192 × 48 Ny. a: Representative Cartesian frames with least aliasing and most aliasing reconstructed with the LL80% and LL95% filters. b: Representative PR frames with least aliasing and most aliasing reconstructed with the LL80% and LL95% filters. Greater aliasing results from the use of the filter with a greater temporal frequency passband, as expected. The aliasing (shown by arrows) presented here was due to breathing or catheter motions, and appeared in synchrony with the respiratory cycle. In frames in which aliasing was greatest, the aliasing with PR was less obstructive.
The average image quality score, as assessed by the interventionalist, changed from 2.9 (artifacts, but images are interpretable) without rFOV to 2.1 (artifacts, but not in the region of importance) with rFOV, demonstrating measurable image quality improvement.
DISCUSSION AND CONCLUSIONS
This investigation demonstrates that rFOV processing is successful for static objects in the FOV. For any real imaging situation, there is a mixture of static and dynamic objects within the FOV, and therefore it is always helpful to perform rFOV processing with PR.
For interventional MRI, long periods of continuous scanning are required for guidance of interventions, thus facilitating rFOV techniques that employ temporal filtering. rFOV provides reduced temporal blur compared to view-sharing (Fig. 5), and improved artifact reduction compared to unfiltered imaging (Fig. 6). The reduced sensitivity of PR to a potentially dynamic outer FOV, as compared to Cartesian rFOV (Fig. 7), is valuable for interventional imaging because scan-plane changes and breathing and catheter movements cause situations in which the entire FOV changes significantly and frequently. In imaging experiments of static phantoms, rFOV processing provided artifact suppression roughly equivalent to doubling the number of projections (Fig. 2). For PR, stationary objects enjoy a 2 Np PSF, while dynamic objects are represented with a PSF of Np projections (Fig. 4). Although rFOV imaging in general cannot be applied to a wholly dynamic FOV because artifacts and image degradation occur (Fig. 4) (9,24), rFOV does improve image quality significantly for PR even with a wholly dynamic FOV. Small FOVs can be prescribed and any slice plane rotation can be interactively requested without concern for obstructive aliasing. It is possible to use a filter with a larger passband for PR rFOV compared with Cartesian rFOV (Fig. 7), and therefore obtain greater temporal resolution (Fig. 5). PR combined with rFOV increases temporal resolution roughly twofold, which greatly improves visualization of rapid nonrepetitive motions that occur during interventions.
ACKNOWLEDGMENTS
The authors thank Scott Smith and Ken Larson of Boston Scientific for the catheters used in this study, and J. Andrew Derbyshire of NIH for helpful insights.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- 1.Rasche V, Holz D, Kohler J, Proksa R, Roschmann P. Catheter tracking using continuous radial MRI. Magn Reson Med. 1997;37:963–968. doi: 10.1002/mrm.1910370623. [DOI] [PubMed] [Google Scholar]
- 2.Peters DC, Lederman RJ, Dick AJ, Raman VK, Guttman MA, Derbyshire JA, McVeigh ER. Interactive undersampled projection reconstruction for active catheter imaging, with adaptable temporal resolution and catheter-only views. Magn Reson Med. 2003;49:216–222. doi: 10.1002/mrm.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spuentrup E, Ruebeen A, Schaeffter T, Manning WJ, Gunther RW, Buecker A. Magnetic resonance-guided coronary artery stent placement in a swine model. Circulation. 2002;105:874–879. doi: 10.1161/hc0702.104165. [DOI] [PubMed] [Google Scholar]
- 4.Bucker A, Neuerburg JM, Adam GB, Glowinski A, Schaeffter T, Rasche V, van Vaals JJ, Gunther RW. Real-time MR guidance for inferior vena cava filter placement in an animal model. J Vasc Interv Radiol. 2001;12:753–756. doi: 10.1016/s1051-0443(07)61448-1. [DOI] [PubMed] [Google Scholar]
- 5.Weiss S, Rasche V. Projection-reconstruction reduced [correction of reduces] FOV imaging. Magn Reson Imaging. 1999;17:517–525. doi: 10.1016/s0730-725x(98)00212-4. [DOI] [PubMed] [Google Scholar]
- 6.Scheffler K, Hennig J. Reduced circular field-of-view imaging. Magn Reson Med. 1998;40:474–480. doi: 10.1002/mrm.1910400319. [DOI] [PubMed] [Google Scholar]
- 7.Larson A, Simonetti OP. Using UNFOLD to remove radial streak artifact in undersampled projection reconstruction cine imaging; Proceedings of the 10th Annual Meeting of ISMRM; Honolulu. 2002. p. 77. [Google Scholar]
- 8.Peters DC, Ennis DB, McVeigh ER. 3D breath-held SSFP cardiac MR with projection reconstruction.; Proceedings of the 10th Annual Meeting of ISMRM; Honolulu. 2002. p. 1625. [Google Scholar]
- 9.Madore B, Glover GH, Pelc NJ. Unaliasing by Fourier-encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn Reson Med. 1999;42:813–828. doi: 10.1002/(sici)1522-2594(199911)42:5<813::aid-mrm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Parrish TB, Hu X. Hybrid technique for dynamic imaging. Magn Reson Med. 2000;44:51–55. doi: 10.1002/1522-2594(200007)44:1<51::aid-mrm9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Fredrickson JO, Pelc NJ. Temporal resolution improvement in dynamic imaging. Magn Reson Med. 1996;35:621–625. doi: 10.1002/mrm.1910350425. [DOI] [PubMed] [Google Scholar]
- 12.Kellman P, Sorger JM, Epstein FH, McVeigh ER. Low-latency temporal filter design for real-time MRI using UNFOLD. Magn Reson Med. 2000;44:933–939. doi: 10.1002/1522-2594(200012)44:6<933::aid-mrm15>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madore B. Using UNFOLD to remove artifacts in parallel imaging and in partial-Fourier imaging. Magn Reson Med. 2002;48:493–501. doi: 10.1002/mrm.10229. [DOI] [PubMed] [Google Scholar]
- 14.Peters DC, Korosec FR, Grist TM, Block WF, Holden JE, Vigen KK, Mistretta CA. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43:91–101. doi: 10.1002/(sici)1522-2594(200001)43:1<91::aid-mrm11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Peters DC, Epstein FH, McVeigh ER. Myocardial wall tagging with undersampled projection reconstruction. Magn Reson Med. 2001;45:562–567. doi: 10.1002/mrm.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale B, Wendt M, Duerk JL. A rapid look-up table method for reconstructing MR images from arbitrary k-space trajectories. IEEE Trans Med Imaging. 2001;20:207–217. doi: 10.1109/42.918471. [DOI] [PubMed] [Google Scholar]
- 17.Quick HH, Kuehl H, Kaiser G, Hornscheidt D, Mikolajczyk KP, Aker S, Debatin JF, Ladd ME. Interventional MRA using actively visualized catheters, TrueFISP, and real-time image fusion. Magn Reson Med. 2003;49:129–137. doi: 10.1002/mrm.10334. [DOI] [PubMed] [Google Scholar]
- 18.Guttman MA, Lederman RJ, Sorger JM, McVeigh ER. Real-time volume rendered MRI for interventional guidance. J Cardiovasc Magn Reson. 2003;4:431–442. doi: 10.1081/jcmr-120016382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aksit P, Derbyshire JA, Serfaty JM, Atalar E. Multiple field of view MR fluoroscopy. Magn Reson Med. 2002;47:53–60. doi: 10.1002/mrm.10035. [DOI] [PubMed] [Google Scholar]
- 20.Oppenheim A, Schafer R, Buck J. Digital signal processing. Prentice Hall; Upper Saddle River: 1999. p. 354. [Google Scholar]
- 21.Shankaranarayanan A, Simonetti OP, Laub G, Lewin JS, Duerk JL. Segmented k-space and real-time cardiac cine MR imaging with radial trajectories. Radiology. 2001;221:827–836. doi: 10.1148/radiol.2213010455. [DOI] [PubMed] [Google Scholar]
- 22.Lederman RJ, Guttman MA, Peters DC, Thompson RB, Sorger JM, Dick AJ, Raman VK, McVeigh ER. Catheter-based endomyocardial injection with real-time magnetic resonance imaging. Circulation. 2002;105:1282–1284. doi: 10.1161/01.CIR.0000012425.71261.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttman MA, Dick AJ, Lederman RJ, Sorger JM, Raman VK, Kellman P, McVeigh ER. Accelerated cardiac interventional imaging with real-time display; Proceedings of the 10th Annual Meeting of ISMRM; Honolulu. 2002. p. 2250. [Google Scholar]
- 24.Di Bella EVR, Wu YJ, Alexander AL, Parker DL, Green D, McGann CJ. Comparison of temporal filtering methods for dynamic contrast MRI myocardial perfusion studies. Magn Reson Med. 2003;49:895–902. doi: 10.1002/mrm.10439. [DOI] [PubMed] [Google Scholar]