Abstract
This article reviews recent preclinical and clinical advances in the use of pretargeting methods for the radioimmunodetection and radioimmunotherapy of cancer. Whereas directly-labeled antibodies, fragments, and subfragments (minibodies and other constructs) have shown promise in both imaging and therapy applications over the past 25 years, their clinical adoption has not fulfilled the original expectations due to either poor image resolution and contrast in scanning or insufficient radiation doses delivered selectively to tumors for therapy. Pretargeting involves the separation of the localization of tumor with an anticancer antibody from the subsequent delivery of the imaging or therapeutic radionuclide. This has shown improvements in both imaging and therapy by overcoming the limitations of conventional, or 1-step, radioimmunodetection or radioimmunotherapy. We focus herein on the use of bispecific antibodies followed by radiolabeled peptide haptens as a new modality of selective delivery of radionuclides for the imaging and therapy of cancer. Our particular emphasis in pretargeting is the use of bispecific trimeric (3 Fab’s) recombinant constructs made by a modular method of antibody and protein engineering of fusion molecules called Dock and Lock (DNL).
Keywords: Bispecific antibody, cancer, Dock and Lock, fusion proteins, monoclonal antibodies, molecular imaging, pretargeting, radioimmunodetection, radioimmunotherapy, radionuclides
Introduction
The fundamental challenge for imaging is achieving a high signal over adjacent normal tissues, resulting in high image resolution. In therapy, the challenge is the delivery of tumoricidal doses of the therapeutic agent while sparing normal tissues from unacceptable toxicities. When using antibodies or fragments to target radioactivity to tumors, the binding specificity, pharmacokinetics and biodistribution have to be matched to the radionuclide, tumor, and disease setting for optimal results to be achieved. The nature and size of the immunoglobulin, or its smaller constructs, will determine how quickly it reaches the target antigen and clears from the blood, and the extent, penetration, and duration of its binding to the tumor vs. normal tissues.
IgG, which is the principal antibody form used, clears very slowly from the blood, requiring several days before a sufficient amount leaves the circulation to achieve a specific concentration in the tumor vs. blood and adjacent tissue radioactivity. Its slow clearance is in part due to its large size, ~150 kD, which impedes its extravasation, resulting in a slow tumor accretion (maximum tumor uptake achieved within 1–3 days). In murine-human tumor xenograft models, uptake is typically between 10–30% of the injected activity per gram tumor, but in humans, with a larger vascular and extravascular volume of distribution, this accretion is reduced to much less-- 0.1% per gram.1, 2 As the molecular size of an antibody is reduced from a divalent F(ab′)2 fragment (~100 kD) to the monovalent binding Fab′ fragment (~50 kD), there is a progressively faster clearance from the blood, and the maximum tumor uptake is achieved more quickly and often with better tumor penetration, but this at the cost of having proportionally less of the injected product reaching the tumor and with a commensurately shorter residence time. 1, 3, 4 Molecular engineering has provided even smaller antibody structures, such as scFv (~25 kD), which are cleared more rapidly from the blood, and have an even lower uptake and shorter retention in tumors. However, the rapid clearance of these molecules from the blood and adjacent, antigen-negative tissues, can result in early, high tumor-to-background ratios, achieving relatively strong signals compared to background.5–8
A number of innovative strategies have been undertaken to restore the multivalency of an antibody, so as to overcome this deficiency and enhance tumor retention. For example, by deleting the CH2 sequence of an IgG, the resulting construct, while still divalent and nearly 100 kD in size, clears extraordinarily fast from the blood, resulting in high localization ratios within a short period of time.9 New constructs, ranging from divalent diabodies, minibodies, (scFv)2-Fc, and other assorted constructs, are essentially composed of multiple scFv’s tethered in different ways, as illustrated in Figure 1.6–8, 10
Figure 1.

Schematic representation of several molecular constructs of antibodies. By splicing the Vh and VL of an IgG’s heavy and light chains (Fv-portion) and linking these polypeptide chains with a linker that is generally 15–18 amino acids in length, the 2 chains will self-associate to form the monovalent scFv (single chain Fv). Progressively shortening the length of the amino acid linker will result in various forms of constructs, such as diabodies to tetrabodies, with multivalent binding ability. Another construct simply removes the CH2 domain that otherwise maintains the parent IgG’s divalent binding structure, but has markedly faster clearance from the blood. The (scFv)2-Fc is another larger construct with fast blood clearance properties due to modifications made in amino acids that are involved in FcRN-binding. The minibody is another example of a fast clearing, smaller engineered construct with divalent antigen binding
Despite the fact that certain aspects of their targeting properties are not always optimal, directly-labeled antibodies still provide certain advantages. For example, in radioimmunodetection, these could differentiate tumor from non-specific proliferation, such as inflammation, when compared to 18F-fluorodeoxyglucose (FDG), or when FDG is not suitable, such as in prostate cancer.11–15 In radioimmunotherapy, evidence for efficacy could be shown in locoregional applications,16–23 in an adjuvant setting.24, 25 or in patients with lymphomas.26–30 Responses in lymphoma are most certainly related to the radiosensitivity of these tumors, because the measured uptake of radiolabeled antibodies in lymphoma is no higher than that in solid tumors.31 However, it is noteworthy that clinical administration of both of the radiolabeled anti-CD20 antibodies that are used commercially involves their combined administration with naked CD20 murine (tositumomab) or chimeric (rituximab) MAb that have anti-tumor activity.32, 33 Perhaps the responses are augmented to some degree by an underlying synergy between the unconjugated and radioconjugated antibodies.34 Indeed, objective responses have been observed in lymphoma patients given radiolabeled antibodies having low radioactivity or low doses of antibody, and in some cases without visual targeting to a given lesion. Most of the antibodies used as radioconjugates for solid tumors have not been as effective as in lymphomas, so that a variety of approaches are being undertaken to enhance the therapeutic response.31
Pretargeting
As indicated above, the single most challenging problem with the delivery of antibody-targeting radionuclides is that the efficiency of selective targeting is relatively poor. While manipulating antibody forms to better match the physical properties of readily available radionuclides can enhance a certain targeting property, there is an inevitable trade-off that has continued to hinder visualization or limit the maximum absorbed radiation dose to less than optimal levels.31 A targeting strategy that preserves the highest tumor accretion observed with a directly radiolabeled IgG, while eliminating the untargeted radioactivity rapidly from the blood and normal tissues so as to minimize toxicity, would be an acceptable alternative strategy.
The basic framework for this alternative targeting strategy can be found in the technology known as pretargeting. Nearly 20 years ago, investigators facing the dilemma of having to match the targeting properties of an antibody with a radionuclide concluded that it would be better to separate the antibody targeting moiety from the radiolabeled effector, administering the radionuclide only after optimal pretargeting by the antibody has occurred. To accomplish this, targeting was first achieved with a bispecific antibody (bsMAb) that binds both to a target antigen as well as to a radiometal-chelate complex (i.e., the effector molecule).35, 36 Over the years, several other pretargeting methods have evolved, particularly involving biotin-avidin (or streptavidin) binding, with encouraging results in certain situations.37 Figure 2 reviews these approaches and Table 1 lists the major issues surrounding the use of the streptavidin/biotin- and bsMAb-based pretargeting methods, as discussed previously.38, 39
Figure 2.
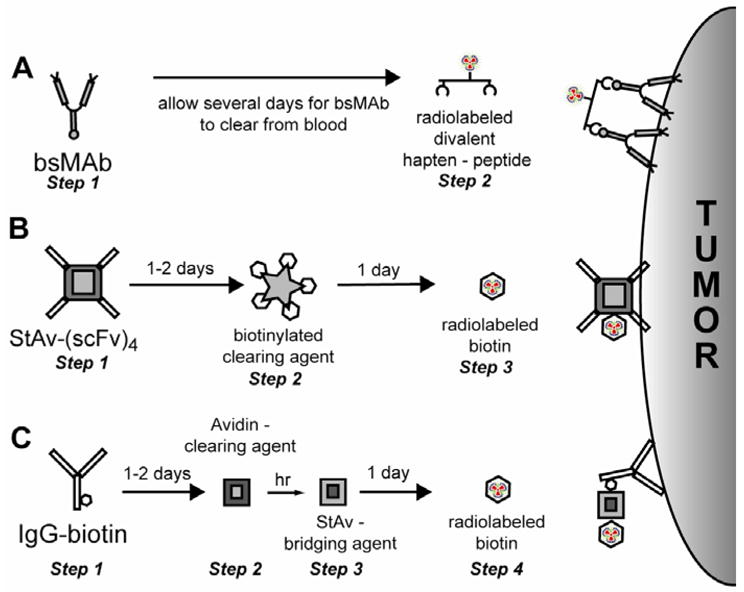
Pretargeting methods used clinically. (A) For the bispecific antibody (bsMAb) method, after allowing time for the bsMAb to localize the tumor and clear from the blood, a radiolabeled divalent hapten-peptide is given. Within minutes the hapten-peptide is available for tumor binding, while the vast majority clears from the blood. (B) Conventionally known as the “2-step” streptavidin-pretargeting approach, this method utilizes a streptavidin-scFv fusion protein. It is given 1–2 days to target the tumor, and then the excess in the blood is removed by the administration of a clearing/blocking agent. The complexes are cleared into the liver, and then 1-day later, radiolabeled biotin is given, which very rapidly localizes in the tumor while also quickly clearing from the body. (C) The “3 step” pretargeting method starts with the injection of an IgG-biotin conjugate. After localizing in the tumor, the excess conjugate is removed from the blood using avidin, which clears the conjugate to the liver. A few hours later, streptavidin is given. Streptavidin will bind to the biotin conjugate in the tumor and by the next day, it will have cleared from the blood. Because streptavidin is multivalent, the radiolabeled biotin given the next day can be localized in the streptavidin bound to the IgG-biotin conjugate in the tumor.
Table I.
Overview of streptavidin/avidin- and bsMAb-based pretargeting systems
| Streptavidin/avidin-biotin | bsMAb-hapten |
|---|---|
Advantages
|
Advantages
|
Disadvantages
|
Disadvantages
|
- Retention in the target for both procedures is ultimately governed by the affinity of the antibody for the target antigen.
- Pretargeting systems are best applied for targets that would not be internalized rapidly as a consequence of the first antibody-binding step.
Testing in animal models has revealed that radionuclide uptake in tumors can be as high as that measured with a directly radiolabeled IgG, but with maximum accretion occurring within minutes rather than several hours or even days.40, 41 Importantly, the radionuclide is cleared very rapidly from the body, with more than 80% of the product eliminated in the urine within a few hours, allowing tumor/blood and tissue ratios to often be ≥10:1 within 1 hour of the radionuclide injection. Tissue retention of the radiolabeled effector molecule is very low, even in the kidneys and liver, tissues that very often have elevated uptake of directly radiolabeled antibodies, unless radioiodine is used.31 Collectively, these properties have significantly enhanced both the imaging and therapeutic utility of these pretargeting procedures as compared to directly radiolabeled antibodies.37, 40, 42 The focus of this review is to discuss the use of bsMAbs in a pretargeting scheme.
The Rationale of the Affinity Enhancement System (AES)
A few years after the initial concept of reversible carriers for drugs and radionuclides was introduced by Goodwin et al.,35 this technique had begun to show promising imaging of patients with colorectal tumors, using a radiolabeled hapten pretargeted by a bispecific anti-CEA x anti-hapten antibody.43 Even at this time, laboratory studies had progressed to where substantial targeting improvements were observed by replacing the monovalent hapten used in the initial pretargeting studies with a bivalent one. Referred to as the affinity enhancement system (AES),44 the bivalent radiolabeled hapten conceivably could bind simultaneously to two neighboring bispecific antibody molecules bound to the target tumor cell, which conferred a longer residence time in the tumor, while binding to excess bsMAb in the circulation would be rapidly reversible, making a clearance step unnecessary. Surprisingly, simple bivalent haptens with relatively short connecting chains, such as tyrosyl-lysine coupled on both the alpha-NH2 of tyrosine and the epsilon-NH2 of lysine to DTPA, were able to bind simultaneously to two anti-hapten antibody molecules. Convincing evidence that cooperative binding at target surfaces occurs was provided by the use of a couple of haptens (e.g., DNP and DTPA-indium, or DTPA-indium and histamine-succinyl-glycine, HSG), covalently linked together and carrying radioactivity, and pretargeted with two distinct bispecific antibodies, each one recognizing a given target antigen and one of the haptens. Target cells expressing both antigens bound much more asymmetric bivalent hapten when incubated with a mixture of the bsMAbs than with only one of them, and target cells expressing only one antigen bound much less hapten, even in the presence of the mixture of bsMAbs. This was shown both in vitro and in vivo by targeting spleen cells in CBA/N mice (I-Ek and Lyb-8.2 (mouse CD22-positive), as compared to AKR/N (Lyb-8.2-negative) mice, BALB/c (I-Ek-negative) or DBA/2 (I-Ek and Lyb-8.2-negative) mice, and in human Burkitt lymphoma cells (Ramos) that express both CD10 and CD20 antigens.45, 46 These findings were confirmed independently,47, 48 thus making the divalent hapten an integral part of a bsMAb-based pretargeting system.
Antibodies are naturally monospecific, and therefore bsMAbs reactive with a tumor antigen and the effector hapten need to be prepared for pretargeting in one of three ways. The first generation of bsMAbs was produced chemically. Most commonly, Fab′ fragments were generated, which naturally had at least one free sulfhydryl group that could be derivatized so that, when added to another Fab′-SH, a stable thio-ether linkage was formed. This coupling approach has the advantage of orienting the 2 proteins in a very specific manner. F(ab′)2 and IgG’s of anti-tumor antibodies also have been modified by a similar chemical coupling approach, with the anti-hapten Fab′-SH being added in a more random orientation.49 Secondly, bsMAbs can be produced in prokaryotic or eukaryotic expression vectors using recombinant DNA technology (discussed below). Finally, bsMAbs can be produced by the fusion of two hybridoma cell lines producing the two parental monoclonal antibodies. The resulting quadroma cell line expresses the heavy and light chains of both parental MAbs. These heavy and light chains assemble randomly, theoretically resulting in twelve different ‘immunoglobulin-like molecules,’ of which only one is the functional bsMAb.
Early Studies with AES Pretargeting for Radioimmunodetection and Radioimmunotherapy
Experimental Studies
When compared to direct targeting with 111In-labeled F(ab′)2 in nude mice bearing melanoma xenografts,50 a bsMAb-pretargeted radiolabeled hapten provided higher tumor-to-blood ratios within just three hours after the radiolabeled hapten injection, tumor to liver and kidney ratios were improved 7 to 10 times, and remarkably, tumor uptake was comparable to that of the 111In-F(ab′)2, even though the radiolabeled hapten cleared from the blood much faster than the 111In-F(ab′)2. For therapy, studies in mice bearing human colorectal tumor xenografts predicted that AES-bsMAb pretargeting could deliver 31.8 Gy/mCi (0.86 Gy/MBq) to tumors, with a tumor-to-blood dose ratio higher than 6:1.51 Further experiments demonstrated that pretargeted radioimmunotherapy could suppress tumor growth for up to 8 months and cure 33% of the treated animals in a mouse model of established human colorectal cancer.52 In the same model, the directly labeled anti-CEA F(ab′)2 fragment showed little efficacy. Additional studies were performed using a chemically conjugated humanized anti-CEA x murine anti-(In)DTPA bsMAb in nude mice, using a di-DTPA-peptide that contained a binding ligand suitable for 99mTc (imaging) or 188Re/186Re (therapy), and while confirming the exceptional ability to target radionuclides, it also expanded the repertoire of radionuclides that could be used in this system.53, 54
In contrast to the chemically conjugated F(ab′)2-like bsMAbs that were commonly used, the group in Nijmegen examined pretargeting with bsMAbs produced by quadroma technology. The hapten-binding arm of the bsMAb was derived from a monoclonal antibody, DTIn-1, which reacted specifically with DTPA loaded with indium (Kd = 0.23 nM).55 The hybridoma cells producing the DTIn-1 antibody were fused somatically with hybridoma cells producing the anti-renal cell carcinoma (RCC) antibody G250 (anti-carbonic anhydrase IX, Kd = 0.25 nM). The 17.56 quadroma cell line stably produced G250 x DTIn-1 bsMAb IgG that was reactive with G250 and with the DTPA-In hapten.55 The quadroma cells produced a mixture of the immunoglobulin-like molecules, with the functional bsMAb comprising 8–15% of the Protein-A-absorbed IgG fraction, requiring additional affinity chromatography and ion-exchange purification.55, 56
The pretargeting strategy using the G250 x DTIn-1 bsMAb IgG was optimized with respect to the structure of the hapten, bsMAb dose, the use of a blocking agent, and the time interval between the two injections using athymic nude mice bearing RCC xenografts.48, 57 Initial studies examining the targeting of 111In-DTPA (monovalent hapten) in RCC-bearing animals pretargeted with the G250 x DTIn-1 IgG bsMAb (2–50 μg) 3 days earlier revealed a maximum tumor uptake of 111In-DTPA using 15 μg of the bsMAb. Absolute uptake of 111In-DTPA was low (1–7% ID/g), but the rapid clearance of the radioactivity from the normal tissues resulted in very high tumor/nontumor ratios (e.g., a tumor/blood ratio >300 at 24 h after injection).57 Even though the blood concentration of the bsMAb was still high (10–15% ID/g) at the time 111In-DTPA was given, 111In-DTPA cleared rapidly from the blood, indicating that bsMAb-111In-DTPA complexes formed in the blood could easily dissociate, making the use of a clearing agent superfluous. The most important parameter determining the accumulation of activity in the tumor using this pretargeting system was the form in which the radiolabeled hapten was administered. For example, in mice bearing subcutaneous NU-12 tumors, uptake of the pretargeted 111In-diDTPA-FKYK (a tetrapeptide conjugated with two DTPA moieties) in the tumor was extremely high (77.5 ± 40.8% ID/g, 4 h post-injection) as compared to the monovalent DTPA-111In (2.24 ± 0.66% ID/g, 4 h post-injection.).48 More importantly, the bivalent chelate was virtually completely retained in the tumor over several days (tumor uptake: 90.5 ± 37% ID/g, 72 h post-injection) (Figure 3). The bivalent peptide cleared from the blood and all other normal tissues very efficiently. Imaging of the mice confirmed the rapid tumor accretion of the 111In-labeled bivalent peptide, as well as the effective retention of the radiolabel in the tumor (Figure 4).
Figure 3.
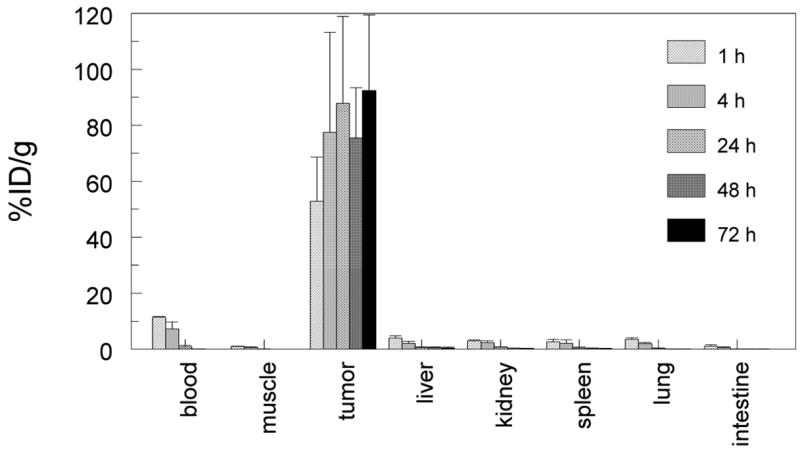
Biodistribution of 111In-di-DTPA-FKYK hapten-peptide at 1, 4, 24. 48 and 72 h after injection with radiotracer in mice bearing subcutaneous NU-12 tumors that were pretargeted 3 days earlier with 15 μg G250 x DTIn-1 bsMAb.
Figure 4.
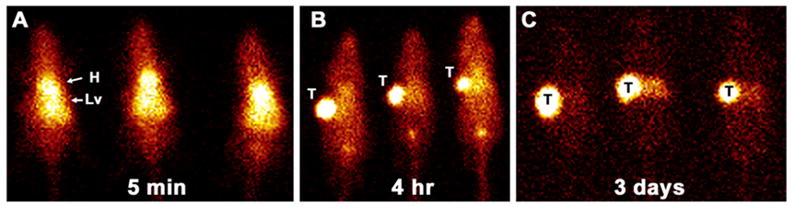
Scintigraphic images of three nude mice bearing NU-12 tumors in the upper left flank that were given 15 μg of G250 x DTIn-1 bsMAb and then 3 days later received 50 μCi di-DTPA-FKYK hapten-peptide. Imaging was performed at 5 min (A), 4 hours (B) and 3 days (C) after the radiolabeled diDTPA-peptide was given.
Although this system was suitable for pretargeting an 111In-labeled peptide, the real challenge in pretargeting is to make this strategy suitable for a wide array of radionuclides. Unfortunately, the DTIn-1 anti-hapten antibody does not bind to a DTPA moiety that can stably chelate other radiometals, such as 90Y or 177Lu, thereby limiting its use. However, the tyrosine residue in the diDTPA-FKYK peptide allowed it to be radioiodinated. Interestingly, unlike 111In-diDTPA-FKYK peptide, the radioiodinated product was not retained well in the tumor. To improve the residence time of the radioiodinated product in the tumor, a new peptidase-resistant diDTPA D-amino acid peptide was synthesized. Tumor-bearing nude mice were pretargeted with the G250 x DTIn-1 bsMAb, followed 72 h later with either the 125I- or 111In-labeled L- or D-amino acid di-FKYK. This showed that the tumor uptake and retention of the 125I-labeled D-peptide was similar to its 111In-D- and L-labeled counterpart, whereas that of the 125I-L-peptide was lower (Figure 5).58 This study indicated that the uptake and retention of the radioiodinated peptide in the tumor following pretargeting with a bsMAb could be improved significantly by stabilizing the peptide with D-amino acids.
Figure 5.
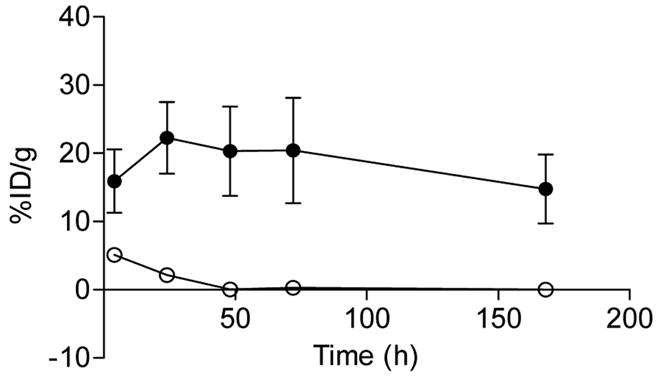
Uptake and retention of the 125I- L-amino acid d-DTPA-peptide (○) and 125I-D-amino acid di-DTPA-peptide (●) in SK-RC-52 tumors. Tumor bearing mice received the radiolabeled peptides 72 h after pretargeting tumors with 15 μg bsMAb G250 x DTIn-1 bsMAb (mean ± SD).
As indicated earlier, the specificity of the anti-hapten-binding arm can place certain limitations on the selection of compounds used in bsMAb pretargeting. The pretargeting of radionuclides has most often been facilitated by having an anti-hapten antibody developed against a chelate capable of binding a radionuclide, such as the anti-indium-loaded DTPA antibodies mentioned earlier. As long as two (In)-DTPA moieties are included in an effector to enhance binding, conceivably the effector could be modified with other binding ligands to broaden the types of materials attached. However, since DTPA can bind a number of different metals, even if a more appropriate ligand were present in the effector molecule for binding a specific radiometal, it is possible that some of the radiometal would be bound less stably to the DTPA. In order to avoid this competition, the hapten portion of the effector for binding the bsMAb should not itself be involved in the radionuclide-loading process. A binding system meeting this specification was first described by Janevik-Ivanovska et al.58 and later by Sharkey et al.59 The hapten is a synthetic derivative of histamine, histamine-succinyl-glycine (HSG), which binds to the monoclonal antibody, 679, with an affinity of <10−9 M, but with low (i.e., 10−4 M) binding to histamine.60 Hapten-peptides have been developed for use in this binding system with radionuclides such as 111In, 90Y, 177Lu, 99mTc, 188Re, 67Ga/68Ga,131I, and 124I, 59, 61–63 but conceivably other ligands capable of binding fluorescent or magnetic compounds, or even cytotoxic chemicals, could be adapted, so long as binding to the divalent-HSG moiety is preserved within such structures.
Clinical Studies
AES pretargeting was first introduced clinically in the early 1990’s as an imaging tool, prior to the development of FDG. The bsMAb was a chemical conjugate between the Fab fragment of an anti-CEA antibody and the Fab fragment of an anti-DTPA-indium antibody, and the hapten was di-DTPA-tyrosyl-lysine, labeled with 111In. Initial clinical studies showed the feasibility of tumor detection in primary colon cancer64, 65 and medullary thyroid carcinoma (MTC),66 showing favorable tumor uptake (1.8–17.5 % injected dose/kg in colorectal cancer and 2.75–139 % injected dose/kg in MTC patients) 48 h post-injection, and with high specificity. Tumor detection by AES immunoscintigraphy also was achieved in metastatic colon64 and lung cancers.67 In MTC, circulating calcitonin signaled the presence of tumor after radical surgery. In a subsequent clinical trial in this disease, AES pretargeted radioimmunodetection revealed occult lesions in 21/29 patients (72%), with only 2 false-positives.68
The high tumor-to-normal tissue ratios observed in these clinical studies prompted a preliminary dosimetry investigation in small-cell lung cancer (SCLC) and MTC.69 In this study, the hapten was labeled with 131I and absorbed doses, calculated for tumors, were 4.2 to 174 cGy/mCi (1.1 to 47 Gy/GBq) in patients with MTC and 1.7 to 8 cGy/mCi (0.5 to 2.1 Gy/GBq) in patients with small-cell lung cancer. Thus, dosimetry in tumor and normal tissues were predictive of favorable results for radioimmunotherapy.
The first radioimmunotherapy phase I/II clinical trial with the bsMAb-AES technique was performed between 1996 and 1998.70 Doses of the anti-CEA x anti-DTPA-indium bsMAb and of 131I-labeled bivalent hapten were escalated in parallel, with a constant ratio of 10 mg bsMAb for 740 MBq (20 mCi) of hapten injected 4 days apart. A total of 26 patients with recurrences of MTC, documented by imaging and a rise in serum calcitonin, were enrolled. Dose-limiting toxicity was hematological, and the maximum tolerated activity was 48 mCi/m2 (1.3 GBq/m2), with grade 3/4 hematological toxicity observed in seven patients, most of them with bone/bone marrow metastases. Tumor absorbed doses ranged from 2.91 to 184 cGy/mCi (0.8 to 50 Gy/GBq). Among the 17 assessable patients, 4 had pain relief, 5 minor responses as measured by CT, and 4 biological responses with a decrease of serum calcitonin levels.
Another phase I/II trial was performed in SCLC patients with CEA-producing tumors using the same reagents and dosing protocol.71 Fourteen patients with proven relapse after chemotherapy were treated with bsMAb-pretargeted radioimmunotherapy, receiving 1.48 to 6.66 GBq (40 to 180 mCi) of 131I-labeled hapten. All patients received the scheduled treatment and toxicity was mainly hematological, with two cases of grade 2 leukopenia and three cases of grade 3 or 4 thrombocytopenia. The estimated tumor dose was 2.6 to 32.2 cGy/mCi (0.7 to 8.7 Gy/BGq). Interestingly, two partial responses (one almost complete for 3 months), and 1 with disease stabilization of more than 24 months, were observed. Efficacy and toxicity were dose-related, with a maximal tolerated dose without hematological rescue of 150 mCi (5.6 GBq).
After these first phase-I studies that established the clinical proof-of-concept of pretargeted-AES radioimmunotherapy, it appeared necessary to achieve a methodological optimization to take maximal advantage of this novel technology. In order to decrease immunogenicity, a chimeric bsMAb, prepared by coupling the Fab fragment of the humanized anti-CEA antibody, hMN14,72 to the murine anti-hapten Fab, was used instead of the previous fully murine bsMAb. Optimization was determined in studies that involved 35 patients with CEA-producing tumors, and consisted of 2 phases.73 These studies revealed optimal pretargeting conditions occurred at a bsMAb protein dose of 40 mg/m2, with an interval between the bsMAb and the radiolabeled hapten of 5 days. With these conditions, the mean tumor absorbed dose was 18.5 Gy and tumor-to-whole body, -liver and –kidney mean ratios were 55, 14 and 8.5, respectively. A high bone/bone marrow uptake was observed in 9 patients with advanced MTC, suggesting tumor involvement that was confirmed by MRI in 8 patients.74 Interestingly a meta-analysis of 324 cases in 14 clinical studies found that bone/bone marrow involvement in MTC has been reported to be only 30%.74 This suggests that pretargeted immunoscintigraphy is a more sensitive method for detecting this type of metastatic spread. Hematologic toxicity was dose-limiting, the maximal tolerated dose in advanced MTC patients being reached at 3.1 GBq (84 mCi), while for other CEA-producing tumors (colorectal, lung and pleural), the maximal tolerated dose was not reached at 5.5 GBq (150 mCi). No complete or partial responses were observed in the 22 assessable patients included in these optimization studies, but disease stabilizations, with a duration from 3 to 12+ months, were observed in 41% of patients (64% in patients examined with the 75 mg/m2 bsMAb dose and 22% with the 40 mg/m2 bsMAb dose).75
Recent Results on Efficacy of Pretargeted Radioimmunotherapy in Patients with Advanced MTC
Over the course of several years, a total of 35 patients with advanced MTC were examined in two phase-I clinical studies. Five minor responses, three with a more than 50% decrease of calcitonin serum concentrations, and 12 patients with both morphological and biomarker stabilization, were observed. Interestingly, 6 years after the first trial (using a murine bsMAb) and 3 years after the second study (using a chimeric bsMAb), long-term stabilization was observed in 53% of the patients. To evaluate the impact of such stabilizations on overall survival, it was necessary to take into account that MTC patients, even with progressive disease, can benefit from long periods of survival in the absence of treatment, emphasizing the need for accurate survival predictors.
An evaluation of a group of 65 MTC patients not treated by radioimmunotherapy revealed that a long calcitonin doubling time (CtDT) was a favorable prognostic indicator of survival.76 In the absence of treatment, a high-risk group was defined by a CtDT <6 months and a 5- and 10-year survival of 25% and 8%, respectively. An intermediate-risk group was defined by a CtDT >6 months and <2 years, and a 5-and 10-year survival of 92% and 37%, respectively. Finally, a low-risk group was defined by a CtDT > 2 years, with all patients being alive at the end of the study. Based on these results, all assessable patients treated with pretargeted radioimmunotherapy were stratified in two groups according to CtDT: one high-risk group with a CtDT < 2 years and one low-risk group with a CtDT between 2 and 5 years.
Overall survival in these treated groups was compared with that of a contemporaneous untreated group of 39 patients, using the same stratification as for the treated group. Overall survival was significantly longer in the high-risk, treated group than in the high-risk, untreated group, with a median survival of 110 vs. 61 months (P <0.030). In the treated group, a biologic response was arbitrarily defined as at least a 100% increase (doubling) of the CtDT measured prior to pretargeted radioimmunotherapy. According to this definition, 47% of patients were considered to be biological responders who experienced a significantly longer overall survival than non-responders (median of 159 vs. 109 months; P <0.035), and untreated patients (median of 159 vs. 61 months; P <0.010).77
Surprisingly, treated patients with bone/bone marrow tumor involvement had a significantly longer 10-year overall survival (83%) than patients without such involvement (14%; P <0.023). This unexpected result could be related in part to tumor in bone marrow responding to therapy, because earlier results obtained in animal and clinical studies have shown that disseminated small-size disease, in which higher absorbed doses are observed, was the best clinical indication for radioimmunotherapy.2, 24, 78–80 Indeed, bone/bone marrow tumor involvement is characterized by isolated or clustered tumor cells, and thus may be a favorable clinical setting for radioimmunotherapy.
These were the first results of radioimmunotherapy showing efficacy in terms of a survival benefit in patients with a progressive solid tumor.81 Following these findings, a phase-II clinical trial has been implemented using the chimeric humanized anti-CEA x murine anti-(In)DTPA bsMAb at a dose of 40 mg/m2 and an interval of 5 days, with all patients receiving 1.8 Gbq/m2 radiolabeled hapten injections. Two groups of patients are being accrued, one with progressing metastatic disease and one with progressing occult disease characterized by a CtDT < 2 years and normal CT and MRI imaging.
Recombinant, Humanized, bsMAb Fusion Proteins: The Dock and Lock Technology
Recombinant bsMAbs have been prepared for pretargeting radionuclides,82–84 as well as for numerous other applications.85–92 In contrast to streptavidin-fusion proteins, bsMAbs can be composed entirely of humanized proteins that would reduce their immunogenicity. Rossi et al. have described a number of different constructs, all based on the anti-HSG-hapten binding system, starting with recombinant proteins that have monovalent binding to both the tumor target and the hapten, to other constructs with divalent binding to the tumor target and monovalent binding to the hapten, being expressed in bacterial, yeast, or mammalian expression systems.82–84 Earlier studies with bsMAbs prepared chemically using an anti-CEA IgG, F(ab′)2, or Fab′ as the tumor-binding arm, which was then coupled to an anti-(In)DTPA Fab′, had indicated that conjugates with divalent tumor-binding gave higher tumor uptake of the pretargeted 99mTc-di(In)DTPA-peptide than those with monovalent binding.49 Furthermore, since bsMAb pretargeting systems historically have not relied on a separate clearing step for optimal pretargeting, the faster blood clearance of the F(ab′)2 × Fab′ bsMAb provided an advantage over the larger IgG × Fab′ bsMAb. The anti-hapten binding arm has been designed primarily for monovalent binding, because the affinity enhancement system’s use of 2 haptens in the radiolabeled hapten-peptides strengthens the avidity of its binding at the tumor. A comparison of the pretargeting ability of a bispecific diabody (~50 kD) with monovalent binding to CEA and the hapten to a Fab′ × Fab′ (100 kD) chemically-conjugated bsMAb with identical specificity revealed a preference for the bispecific diabody.84 The bispecific diabody had a more rapid clearance from the blood that not only allowed for the earlier administration of the 111In-labeled hapten-peptide, but its uptake in the tumor was similar to that obtained with the Fab′ × Fab′ bsMAb, with significantly improved tumor/nontumor ratios. Subsequent studies showed a new bsMAb construct with divalent binding to CEA (~80 kD) capable of increasing the amount of radiolabeled hapten-peptide to the tumor, which would be an advantage for therapeutic applications while maintaining high tumor/nontumor ratios.82 Therapy studies in nude mice bearing established human colon cancer xenografts showed improved responses, and even cures, with a 90Y-DOTA-hapten-peptide pretargeted with this bsMAb, as compared to 90Y-DOTA-anti-CEA IgG.62
Further advances in molecular engineering led to the development of another very unique method for preparing bsMAbs, designated the “Dock and Lock” (DNL) procedure.83 With this procedure, 2 Fab anti-tumor binding arms are joined together with a single Fab anti-hapten binding arm to form a highly stable, ~157-kD fusion protein. The technique takes advantage of the specific protein/protein interactions between the regulatory (R) subunits of human cAMP-dependent protein kinase (PKA) and the anchoring domains (AD) of A-kinase anchor proteins (AKAPs). Amino acids 1–44 of human RIIα were fused to the C-terminal end of a humanized anti-CEA antibody Fd chain via a 14-residue flexible peptide linker.83 This 44-amino acid sequence was modified further by engineering a single cysteine residue adjacent to the amino-terminal end. This recombinant protein produced in mammalian cells is designated C-DDD2-hMN-14 anti-CEA. The C-DDD2-hMN-14 fusion proteins naturally associate to form homodimers due to the interaction of the 44-residue amino acid sequence derived from human RIIα, creating a structure with divalent binding to CEA.
When in the dimeric form, the first 23 residues of the DDD-amino acid sequence form a structure that has a natural binding affinity to the anchoring domains (AD) of the AKAP. Thus, the 44-residue peptide used in the C-DDD2-hMN-14 is responsible for both dimerization and docking, hence it is known as the dimerization and docking domain (DDD). In order to take advantage of this natural attraction to form bsMAbs, the anti-hapten Fd binding arm (h679) is fused with a 17-residue amino acid sequence derived from the synthetic peptide AKAP-IS, which was modified further by the addition of cysteine residues to both the amino and carboxyl-terminal ends. After pairing with the cognate light chain, the fusion protein, designated h679-AD2, is formed (Figure 7). When these 2 products are mixed, they come together naturally in a well-defined orientation based on the DDD/AD binding properties. Although in nature, the AD component binds to the dimerized DDD component with affinities ranging from 9 × 10−8 M to 2 × 10−9 M, the strategically placed cysteine residues favor the formation of a covalent disulfide bond between these residues, creating a more highly stable structure. The anti-CEA bsMAb prepared by this procedure, cleared quickly from mice, leaving < 1% of the injected dose in the blood within 16 h. Animals bearing human colonic tumor xenografts that were pretargeted with this construct and a 99mTc-hapten-peptide showed tumor uptake of ~30% ID/g and tumor/blood ratios of 66:1 just 1 hour after the radiotracer injection, providing an example of the exquisite targeting that can be achieved with this pretargeting system. This same process has been used to prepare bsMAb with specificities to CD20, CD22, and an anti-pancreatic cancer MUC-1 for use in pretargeting.
Figure 7.
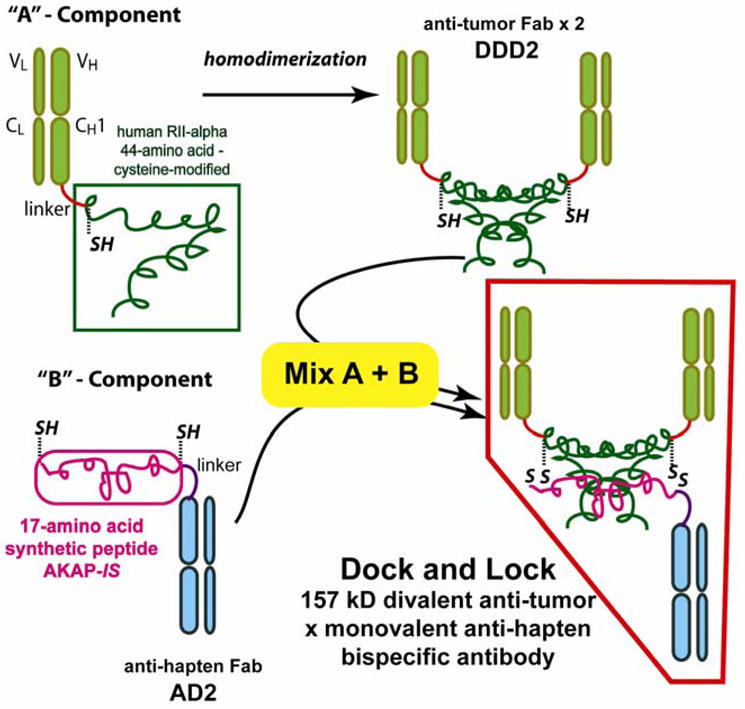
The “Dock and Lock” method for preparing bsMAb starts with the splicing of a cysteine-modified, 44-amino acid sequence to the CH1domain of an anti-tumor antibody using a flexible amino acid linker. This recombinant protein naturally forms well-defined dimers, and is designed the “DDD2 form. A separate recombinant protein is prepared by splicing a synthetic 17-amino acid peptide to the CH1 of an anti-hapten Fab (designated the AD2 form). This peptide is also modified with cysteine in 2 locations. The dimerized DDD2 forms a pocket in which the AD2 will naturally bind (i.e., Dock), but the strategic placement of cysteine allows these structures to form a more stable bond (i.e., Lock).
Although the major emphasis with pretargeted radionuclides has been their prospects for improving therapy with radionuclides, this technique is now being re-discovered as a highly effective imaging method. Today, positron-emission tomography systems combined with FDG have become the leading nuclear imaging method for cancer detection, with sensitivities and specificities that can be as high as 90%.12, 93 However, not all cancers (such as prostate) are as effectively targeted, and there are complications with interpretation, particularly when inflammatory lesions cannot be ruled out. Thus, there are opportunities where the specificity of an antibody imaging method could be advantageous. As mentioned earlier, prior antibody-based imaging methods have suffered from relatively poor image resolution derived from not only low tumor/nontumor ratios, but also low uptake in the tumor. Attempts have been made to devise a variety of constructs with more favorable blood clearance, but while succeeding on this level, often these constructs have low tumor uptake. There is also the difficulty of matching the antibody’s clearance properties with the physical half-life of positron-emitting radionuclides. For example, Cai et al.94 examined targeting of an 18F-labeled anti-CEA diabody in nude mice bearing a human colonic xenograft. Besides having very low radiolabeling yields (<2%), it took more than 1 h for favorable tumor/blood ratios to develop, amounting to ~7:1 within 6 h. Although this report did not include a comparison to 18F-FDG, our experience in the same tumor model has indicated that tumor uptake with FDG is ~2-times higher than that reported for the 18F-diabody, with tumor/blood ratios ~16:1 within 1 h. In contrast, the bsMAb pretargeting method using an 124I-labeled hapten-peptide had nearly a 3-fold higher accretion of the radiolabeled hapten-peptide in the tumor than with FDG, tumor/blood ratios of 9:1 at 1 h and 17:1 by 3 h after the 124I-hapten-peptide injection.63 While microPET imaging studies were able to identify subcutaneous xenografts with both FDG and pretargeting methods, the images were more easily interpreted with pretargeting because of the significantly lower activity in normal tissues and stronger signal intensity in the tumor. More recently, the ability of a 124I-labeled hapten-peptide to localize micrometastatic nodules (≤0.3 mm in diameter) of a human colon cancer cell line growing in the lungs of nude mice highlighted the exquisite sensitivity of this procedure (Figure 8).95 Indeed, even though the tumor cell line in this model had similar FDG uptake in the tumor as the cell line used in the earlier studies, these lesions could not be detected in the lungs by microPET. Necropsy and autoradiographic studies confirmed the superior and specific targeting by the pretargeting method. Thus, with preclinical models illustrating how pretargeting could complement FDG-imaging, and in some instances amplify the detection based on improved specificity, there are now new incentives for re-introducing antibody-based imaging methods in clinical trials to determine their role in cancer management.
Figure 8.
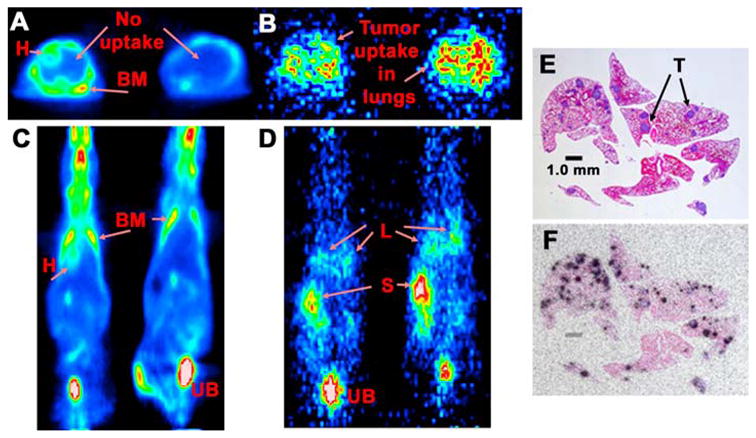
Targeting of a human colonic cancer injected intravenously that grows exclusively in the lungs of nude mice. A (transverse sections through the chest) and C (coronal sections) show the 18F-FDG uptake profile 1.5 h after the injection of 2 nude mice that had ~80 to 120 tumor nodules in the lung, all <0.3 mm in diameter. Uptake is seen in the bone marrow (BM) heart wall (H), and urinary bladder (UB), but no evidence of uptake in the lungs could be detected. B (transverse section through the chest) and D (coronal slice) show the targeting of an 124I-diHSG-peptide 1.5 h after its injection in 2 nude having the same tumor-bearing conditions as the mice in A & C. These mice received an anti-CEA x anti-HSG bsMAb prepared by the Dock and Lock method 1 day earlier. Clear evidence of uptake in the lungs (L) is seen in both sets of images. In the coronal view (D) stomach (S) uptake is observed as a result of dehalogenation of the radioiodine. Kidney uptake was also apparent (not shown). E shows a hematoxylin-eosin-stained section of the lungs taken from a different animal, where the tumor was allowed to grow for 10 additional days. Several purple-stained tumor (T) colonies are seen. This animal was given the anti-CEA bsMAb and one day later received an 111In-labeled di-HSG peptide After 3 hours, the animal was necropsied so that the localization of the radioactivity in the lungs could be revealed by autoradiography. F is an overlay of an autoradiographic film over the stained section illustrating the selective uptake in the tumor colonies.
Conclusions
Highly specific and very sensitive images are possible with a bsMAb pretargeting, and both experimental and clinical studies have shown that pretargeting can have encouraging therapeutic effects. Although the studies indicate that PET imaging will likely outperform SPECT-based imaging systems, it is important to note that the strong signal and high tumor/tissue ratios produce excellent image contrast even with conventional gamma scintillation camera, and thus a pretargeting procedure based on a 99mTc-labeled peptide can be a useful and less expensive alternative to a PET-based system. There are also other positron-emitting radionuclides with preferred PET imaging properties, such as 18F, 64Cu or 68Ga, which might also improve image quality with pretargeting. Thus, humanized bsMAb pretargeting appears to offer new prospects for improved molecular imaging of cancer.
This also appears to be true for radioimmunotherapy, where improved specific targeting and higher tumor radiation doses appear to be feasible. In MTC, initial clinical results with a first-generation pretargeting method have shown evidence of improved survival. Preclinical studies with more advanced pretargeting reagents also have shown superiority over directly-labeled, 1-step, radioimmunotherapy. Therefore, more clinical studies to evaluate these new procedures are needed. Since the reagents for imaging and therapy, except for the final radionuclides chosen, are the same, and integration of both modalities is possible in such trials, we anticipate an eventually broader application of this technology to the management of cancer, and perhaps other diseases.
Figure 6.
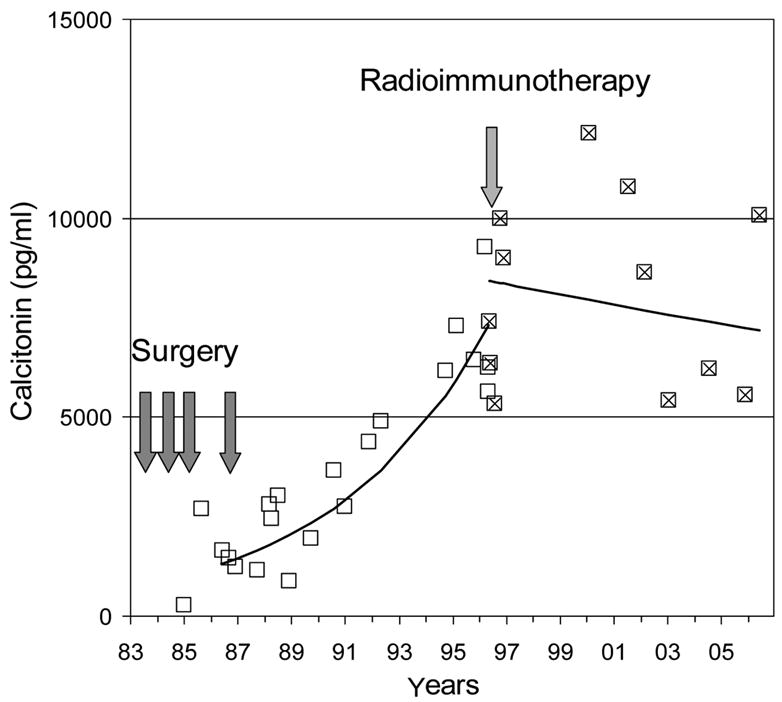
Example of disease stabilization in a patient with MTC treated with AES pretargeted radioimmunotherapy. The patient had undergone 4 different surgeries because of the persistence of calcitonin in blood. After the final surgery, calcitonin continued to increase steadily with a doubling time of 4 years (open boxes). The patient was then enrolled in the first AES radioimmunotherapy clinical trial and treated in May 1996. After radioimmunotherapy, calcitonin serum levels stabilized (“x”-boxes), and the patient’s condition has remained stable ever since. CEA serum levels showed a similar parallel behavior (data not shown).
Acknowledgments
Funding Sources: Drs. Goldenberg and Sharkey are supported in part by USPHS grants P01 CA10395, R01 CA107088 and R01 CA115755 from the NIH. Drs. Chatal and Barbet are supported by the Nuclear Oncology CPER grant (2001–2006) and INCa grant “Cancéropôles en émergence”. Dr. Boerman is supported in part by the Dutch Cancer Society, NKB-KWF grant KUN 2005-3339.
Abbreviations
- AD
anchoring domain
- AES
affinity enhancement system
- AKAP
A-kinase anchor proteins
- bsMAb
bispecific antibody
- CEA
carcinoembryonic antigen
- CtDT
calcitonin doubling time
- DDD
dimerization and docking domain
- DOTA
1,4,7,10-tetraazacyclododecane-N, N′, N″, N‴-tetraacetic acid
- DTPA
diethylenetriaminepentaacetic acid
- DNP
dinitrophenol
- FDG
18F-fluordeoxyglucose
- HSG
histamine-succinyl-glycine
- MTC
medullary thyroid cancer
- NHL
non-Hodgkin’s lymphoma
- PET
positron-emission tomography
- PKA
C-AMP-dependent protein kinase
- SCLC
small-cell lung cancer
- SPECT
single-photon emission computed tomography
Footnotes
Conflict of Interest Statement: Dr. Goldenberg has financial interests in Immunomedics, Inc., and IBC Pharmaceuticals, Inc., which are developing pretargeting products.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchsbaum DJ. Experimental approaches to increase radiolabeled antibody localization in tumors. Cancer Res. 1995;55:5729s–32s. [PubMed] [Google Scholar]
- 2.Siegel JA, Pawlyk DA, Lee RE, et al. Tumor, red marrow, and organ dosimetry for 131I-labeled anti-carcinoembryonic antigen monoclonal antibody. Cancer Res. 1990;50:1039s–42s. [PubMed] [Google Scholar]
- 3.Sharkey RM, Motta-Hennessy C, Pawlyk D, Siegel JA, Goldenberg DM. Biodistribution and radiation dose estimates for yttrium- and iodine-labeled monoclonal antibody IgG and fragments in nude mice bearing human colonic tumor xenografts. Cancer Res. 1990;50:2330–36. [PubMed] [Google Scholar]
- 4.Behr TM, Sgouros G, Stabin MG, et al. Studies on the red marrow dosimetry in radioimmunotherapy: an experimental investigation of factors influencing the radiation-induced myelotoxicity in therapy with beta-, Auger/conversion electron-, or alpha-emitters. Clin Cancer Res. 1999;5:3031s–43s. [PubMed] [Google Scholar]
- 5.Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med. 1998;42:225–41. [PubMed] [Google Scholar]
- 6.Kenanova V, Olafsen T, Williams LE, et al. Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: optimal pharmacokinetics for therapy. Cancer Res. 2007;67:718–26. doi: 10.1158/0008-5472.CAN-06-0454. [DOI] [PubMed] [Google Scholar]
- 7.Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol. 2002;13:603–8. doi: 10.1016/s0958-1669(02)00352-x. [DOI] [PubMed] [Google Scholar]
- 8.Wittel UA, Jain M, Goel A, Chauhan SC, Colcher D, Batra SK. The in vivo characteristics of genetically engineered divalent and tetravalent single-chain antibody constructs. Nucl Med Biol. 2005;32:157–64. doi: 10.1016/j.nucmedbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Slavin-Chiorini DC, Horan Hand PH, Kashmiri SV, Calvo B, Zaremba S, Schlom J. Biologic properties of a CH2 domain-deleted recombinant immunoglobulin. Int J Cancer. 1993;53:97–103. doi: 10.1002/ijc.2910530119. [DOI] [PubMed] [Google Scholar]
- 10.Wu AM. Engineering multivalent antibody fragments for in vivo targeting. Methods Mol Biol. 2004;248:209–25. doi: 10.1385/1-59259-666-5:209. [DOI] [PubMed] [Google Scholar]
- 11.Binz HK, Amstutz P, Pluckthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23:1257–68. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 12.Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 13.Kelloff GJ, Krohn KA, Larson SM, et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin Cancer Res. 2005;11:7967–85. doi: 10.1158/1078-0432.CCR-05-1302. [DOI] [PubMed] [Google Scholar]
- 14.Zalutsky MR. Potential of immuno-positron emission tomography for tumor imaging and immunotherapy planning. Clin Cancer Res. 2006;12:1958–60. doi: 10.1158/1078-0432.CCR-06-0405. [DOI] [PubMed] [Google Scholar]
- 15.Jana S, Blaufox MD. Nuclear medicine studies of the prostate, testes, and bladder. Semin Nucl Med. 2006;36:51–72. doi: 10.1053/j.semnuclmed.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez RD, Huh WK, Khazaeli MB, et al. A Phase I study of combined modality (90)Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002;8:2806–11. [PubMed] [Google Scholar]
- 17.Alvarez RD, Partridge EE, Khazaeli MB, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 18.Goetz C, Riva P, Poepperl G, et al. Locoregional radioimmunotherapy in selected patients with malignant glioma: experiences, side effects and survival times. J Neurooncol. 2003;62:321–8. doi: 10.1023/a:1023309927635. [DOI] [PubMed] [Google Scholar]
- 19.Mahe MA, Fumoleau P, Fabbro M, et al. A phase II study of intraperitoneal radioimmunotherapy with iodine-131-labeled monoclonal antibody OC-125 in patients with residual ovarian carcinoma. Clin Cancer Res. 1999;5:3249s–53s. [PubMed] [Google Scholar]
- 20.Meredith RF, Alvarez RD, Partridge EE, et al. Intraperitoneal radioimmunochemotherapy of ovarian cancer: a phase I study. Cancer Biother Radiopharm. 2001;16:305–15. doi: 10.1089/108497801753131381. [DOI] [PubMed] [Google Scholar]
- 21.Paganelli G, Bartolomei M, Grana C, Ferrari M, Rocca P, Chinol M. Radioimmunotherapy of brain tumor. Neurol Res. 2006;28:518–22. doi: 10.1179/016164106X116782. [DOI] [PubMed] [Google Scholar]
- 22.Reardon DA, Akabani G, Coleman RE, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J Clin Oncol. 2006;24:115–22. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 23.Riva P, Franceschi G, Frattarelli M, et al. 131I radioconjugated antibodies for the locoregional radioimmunotherapy of high-grade malignant glioma--phase I and II study. Acta Oncol. 1999;38:351–59. doi: 10.1080/028418699431438. [DOI] [PubMed] [Google Scholar]
- 24.Liersch T, Meller J, Kulle B, et al. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results. J Clin Oncol. 2005;23:6763–70. doi: 10.1200/JCO.2005.18.622. [DOI] [PubMed] [Google Scholar]
- 25.Zalutsky MR. Targeted alpha-particle therapy of microscopic disease: Providing a further rationale for clinical investigation. J Nucl Med. 2006;47:1238–40. [PubMed] [Google Scholar]
- 26.Dreyling M, Trumper L, von Schilling C, et al. Results of a national consensus workshop: therapeutic algorithm in patients with follicular lymphoma-role of radioimmunotherapy. Ann Hematol. 2007;86:81–87. doi: 10.1007/s00277-006-0207-0. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg DM. The role of radiolabeled antibodies in the treatment of non-Hodgkin’s lymphoma: the coming of age of radioimmunotherapy. Crit Rev Oncol Hematol. 2001;39:195–201. doi: 10.1016/s1040-8428(01)00108-1. [DOI] [PubMed] [Google Scholar]
- 28.Meredith RF. Ongoing investigations and new uses of radioimmunotherapy in the treatment of non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2006;66:S23–9. doi: 10.1016/j.ijrobp.2006.05.068. [DOI] [PubMed] [Google Scholar]
- 29.Meredith RF, Knox SJ. Clinical development of radioimmunotherapy for B-cell non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2006;66:S15–22. doi: 10.1016/j.ijrobp.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 30.Witzig TE. Radioimmunotherapy for B-cell non-Hodgkin lymphoma. Best Pract Res Clin Haematol. 2006;19:655–68. doi: 10.1016/j.beha.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46 (Suppl 1):115S–27S. [PubMed] [Google Scholar]
- 32.Davis TA, Kaminski MS, Leonard JP, et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10:7792–8. doi: 10.1158/1078-0432.CCR-04-0756. [DOI] [PubMed] [Google Scholar]
- 33.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–63. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez MC, Knox SJ. Radiobiology of radioimmunotherapy: targeting CD20 B-cell antigen in non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2004;59:1274–87. doi: 10.1016/j.ijrobp.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin DA, Meares CF, David GF, et al. Monoclonal antibodies as reversible equilibrium carriers of radiopharmaceuticals. Int J Rad Appl Instrum B. 1986;13:383–91. doi: 10.1016/0883-2897(86)90015-2. [DOI] [PubMed] [Google Scholar]
- 36.Reardan DT, Meares CF, Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265–68. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–34. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg DM, Chang CH, Sharkey RM, et al. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:777–80. doi: 10.1007/s00259-002-1089-6. [DOI] [PubMed] [Google Scholar]
- 39.Paganelli G, Chinol M. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:773–76. doi: 10.1007/s00259-002-1090-0. [DOI] [PubMed] [Google Scholar]
- 40.Sharkey RM, Karacay H, Cardillo TM, et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res. 2005;11:7109s–21s. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 41.Axworthy DB, Reno JM, Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci U S A. 2000;97:1802–7. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boerman OC, van Schaijk FG, Oyen WJ, Corstens FH. Pretargeted radioimmunotherapy of cancer: progress step by step. J Nucl Med. 2003;44:400–11. [PubMed] [Google Scholar]
- 43.Stickney DR, Anderson LD, Slater JB, et al. Bifunctional antibody: a binary radiopharmaceutical delivery system for imaging colorectal carcinoma. Cancer Res. 1991;51:6650–5. [PubMed] [Google Scholar]
- 44.Le Doussal JM, Martin M, Gautherot E, Delaage M, Barbet J. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: enhanced divalent hapten affinity for cell-bound antibody conjugate. J Nucl Med. 1989;30:1358–66. [PubMed] [Google Scholar]
- 45.Le Doussal JM, Gautherot E, Martin M, Barbet J, Delaage M. Enhanced in vivo targeting of an asymmetric bivalent hapten to double-antigen-positive mouse B cells with monoclonal antibody conjugate cocktails. J Immunol. 1991;146:169–75. [PubMed] [Google Scholar]
- 46.Hillairet de Boisferon M, Raguin O, Dussaillant M, Rostene W, Barbet J, Gruaz-Guyon A. Enhanced targeting specificity to tumor cells by simultaneous recognition of two antigens. Bioconjug Chem. 2000;11:452–60. doi: 10.1021/bc9901090. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin DA, Meares CF, McTigue M, et al. Pretargeted immunoscintigraphy: effect of hapten valency on murine tumor uptake. J Nucl Med. 1992;33:2006–13. [PubMed] [Google Scholar]
- 48.Boerman OC, Kranenborg MH, Oosterwijk E, et al. Pretargeting of renal cell carcinoma: improved tumor targeting with a bivalent chelate. Cancer Res. 1999;59:4400–5. [PubMed] [Google Scholar]
- 49.Karacay H, Sharkey RM, McBride WJ, et al. Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody’s valency for the tumor target antigen. Bioconjug Chem. 2002;13:1054–70. doi: 10.1021/bc0200172. [DOI] [PubMed] [Google Scholar]
- 50.Le Doussal JM, Gruaz-Guyon A, Martin M, Gautherot E, Delaage M, Barbet J. Targeting of indium 111-labeled bivalent hapten to human melanoma mediated by bispecific monoclonal antibody conjugates: imaging of tumors hosted in nude mice. Cancer Res. 1990;50:3445–52. [PubMed] [Google Scholar]
- 51.Gautherot E, Le Doussal JM, Bouhou J, et al. Delivery of therapeutic doses of radioiodine using bispecific antibody-targeted bivalent haptens. J Nucl Med. 1998;39:1937–43. [PubMed] [Google Scholar]
- 52.Gautherot E, Rouvier E, Daniel L, et al. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and 131I-labeled bivalent hapten. J Nucl Med. 2000;41:480–7. [PubMed] [Google Scholar]
- 53.Karacay H, McBride WJ, Griffiths GL, et al. Experimental pretargeting studies of cancer with a humanized anti-CEA x murine anti-[In-DTPA] bispecific antibody construct and a 99mTc-/188Re-labeled peptide. Bioconjug Chem. 2000;11:842–54. doi: 10.1021/bc0000379. [DOI] [PubMed] [Google Scholar]
- 54.Gestin JF, Loussouarn A, Bardies M, et al. Two-step targeting of xenografted colon carcinoma using a bispecific antibody and 188Re-labeled bivalent hapten: biodistribution and dosimetry studies. J Nucl Med. 2001;42:146–53. [PubMed] [Google Scholar]
- 55.Kranenborg MH, Boerman OC, Oosterwijk-Wakka JC, de Weijert MC, Corstens FH, Oosterwijk E. Development and characterization of anti-renal cell carcinoma x antichelate bispecific monoclonal antibodies for two-phase targeting of renal cell carcinoma. Cancer Res. 1995;55:5864s–67s. [PubMed] [Google Scholar]
- 56.van Schaijk FG, Oosterwijk E, Soede AC, et al. Pretargeting of carcinoembryonic antigen-expressing tumors with a biologically produced bispecific anticarcinoembryonic antigen x anti-indium-labeled diethylenetriaminepentaacetic acid antibody. Clin Cancer Res. 2005;11:7130s–36s. doi: 10.1158/1078-0432.CCR-1004-0006. [DOI] [PubMed] [Google Scholar]
- 57.Kranenborg MH, Boerman OC, Oosterwijk-Wakka JC, de Weijert MC, Corstens FH, Oosterwijk E. Two-step radio-immunotargeting of renal-cell carcinoma xenografts in nude mice with anti-renal-cell-carcinoma X anti-DTPA bispecific monoclonal antibodies. Int J Cancer. 1998;75:74–80. doi: 10.1002/(sici)1097-0215(19980105)75:1<74::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 58.Janevik-Ivanovska E, Gautherot E, Hillairet de Boisferon M, et al. Bivalent hapten-bearing peptides designed for iodine-131 pretargeted radioimmunotherapy. Bioconjug Chem. 1997;8:526–33. doi: 10.1021/bc970083h. [DOI] [PubMed] [Google Scholar]
- 59.Sharkey RM, McBride WJ, Karacay H, et al. A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res. 2003;63:354–63. [PubMed] [Google Scholar]
- 60.Morel A, Darmon M, Delaage M. Recognition of imidazole and histamine derivatives by monoclonal antibodies. Mol Immunol. 1990;27:995–1000. doi: 10.1016/0161-5890(90)90122-g. [DOI] [PubMed] [Google Scholar]
- 61.Griffiths GL, Chang CH, McBride WJ, et al. Reagents and methods for PET using bispecific antibody pretargeting and 68Ga-radiolabeled bivalent hapten-peptide-chelate conjugates. J Nucl Med. 2004;45:30–9. [PubMed] [Google Scholar]
- 62.Karacay H, Brard PY, Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–85. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 63.McBride WJ, Zanzonico P, Sharkey RM, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med. 2006;47:1678–88. [PubMed] [Google Scholar]
- 64.Le Doussal JM, Chetanneau A, Gruaz-Guyon A, et al. Bispecific monoclonal antibody-mediated targeting of an indium-111-labeled DTPA dimer to primary colorectal tumors: pharmacokinetics, biodistribution, scintigraphy and immune response. J Nucl Med. 1993;34:1662–71. [PubMed] [Google Scholar]
- 65.Chetanneau A, Barbet J, Peltier P, et al. Pretargetted imaging of colorectal cancer recurrences using an 111In-labelled bivalent hapten and a bispecific antibody conjugate. Nucl Med Commun. 1994;15:972–80. doi: 10.1097/00006231-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Peltier P, Curtet C, Chatal JF, et al. Radioimmunodetection of medullary thyroid cancer using a bispecific anti-CEA/anti-indium-DTPA antibody and an indium-111-labeled DTPA dimer. J Nucl Med. 1993;34:1267–73. [PubMed] [Google Scholar]
- 67.Vuillez JP, Moro D, Brichon PY, et al. Two-step immunoscintigraphy for non-small-cell lung cancer staging using a bispecific anti-CEA/anti-indium-DTPA antibody and an indium-111-labeled DTPA dimer. J Nucl Med. 1997;38:507–11. [PubMed] [Google Scholar]
- 68.Barbet J, Peltier P, Bardet S, et al. Radioimmunodetection of medullary thyroid carcinoma using indium-111 bivalent hapten and anti-CEA x anti-DTPA-indium bispecific antibody. J Nucl Med. 1998;39:1172–8. [PubMed] [Google Scholar]
- 69.Bardies M, Bardet S, Faivre-Chauvet A, et al. Bispecific antibody and iodine-131-labeled bivalent hapten dosimetry in patients with medullary thyroid or small-cell lung cancer. J Nucl Med. 1996;37:1853–9. [PubMed] [Google Scholar]
- 70.Kraeber-Bodere F, Bardet S, Hoefnagel CA, et al. Radioimmunotherapy in medullary thyroid cancer using bispecific antibody and iodine 131I-labeled bivalent hapten: preliminary results of a phase I/II clinical trial. Clin Cancer Res. 1999;5:3190s–98s. [PubMed] [Google Scholar]
- 71.Vuillez JP, Kraeber-Bodere F, Moro D, et al. Radioimmunotherapy of small cell lung carcinoma with the two-step method using a bispecific anti-carcinoembryonic antigen/anti-diethylenetriaminepentaacetic acid (DTPA) antibody and iodine-131 Di-DTPA hapten: results of a phase I/II trial. Clin Cancer Res. 1999;5:3259s–67s. [PubMed] [Google Scholar]
- 72.Sharkey RM, Juweid M, Shevitz J, et al. Evaluation of a complementarity-determining region-grafted (humanized) anti-carcinoembryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res. 1995;55:5935s–45s. [PubMed] [Google Scholar]
- 73.Kraeber-Bodere F, Faivre-Chauvet A, Ferrer L, et al. Pharmacokinetics and dosimetry studies for optimization of anti-carcinoembryonic antigen x anti-hapten bispecific antibody-mediated pretargeting of Iodine-131-labeled hapten in a phase I radioimmunotherapy trial. Clin Cancer Res. 2003;9:3973S–81S. [PubMed] [Google Scholar]
- 74.Mirallie E, Vuillez JP, Bardet S, et al. High frequency of bone/bone marrow involvement in advanced medullary thyroid cancer. J Clin Endocrinol Metab. 2005;90:779–88. doi: 10.1210/jc.2004-1500. [DOI] [PubMed] [Google Scholar]
- 75.Kraeber-Bodere F, Rousseau C, Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006;47:247–55. [PubMed] [Google Scholar]
- 76.Barbet J, Campion L, Kraeber-Bodere F, Chatal JF. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:6077–84. doi: 10.1210/jc.2005-0044. [DOI] [PubMed] [Google Scholar]
- 77.Chatal JF, Campion L, Kraeber-Bodere F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705–11. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 78.Blumenthal RD, Sharkey RM, Haywood L, et al. Targeted therapy of athymic mice bearing GW-39 human colonic cancer micrometastases with 131I-labeled monoclonal antibodies. Cancer Res. 1992;52:6036–44. [PubMed] [Google Scholar]
- 79.Boerman OC, Sharkey RM, Blumenthal RD, Aninipot RL, Goldenberg DM. The presence of a concomitant bulky tumor can decrease the uptake and therapeutic efficacy of radiolabeled antibodies in small tumors. Int J Cancer. 1992;51:470–5. doi: 10.1002/ijc.2910510322. [DOI] [PubMed] [Google Scholar]
- 80.Sharkey RM, Weadock KS, Natale A, et al. Successful radioimmunotherapy for lung metastasis of human colonic cancer in nude mice. J Natl Cancer Inst. 1991;83:627–32. doi: 10.1093/jnci/83.9.627. [DOI] [PubMed] [Google Scholar]
- 81.Martins RG, Rajendran JG, Capell P, Byrd DR, Mankoff DA. Medullary thyroid cancer: options for systemic therapy of metastatic disease? J Clin Oncol. 2006;24:1653–5. doi: 10.1200/JCO.2005.05.4106. [DOI] [PubMed] [Google Scholar]
- 82.Rossi EA, Chang CH, Losman MJ, et al. Pretargeting of carcinoembryonic antigen-expressing cancers with a trivalent bispecific fusion protein produced in myeloma cells. Clin Cancer Res. 2005;11:7122s–29s. doi: 10.1158/1078-0432.CCR-1004-0020. [DOI] [PubMed] [Google Scholar]
- 83.Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841–6. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossi EA, Sharkey RM, McBride W, et al. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin Cancer Res. 2003;9:3886S–96S. [PubMed] [Google Scholar]
- 85.Shen J, Vil MD, Jimenez X, et al. Single variable domain antibody as a versatile building block for the construction of IgG-like bispecific antibodies. J Immunol Methods. 2007;318:65–74. doi: 10.1016/j.jim.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Asano R, Sone Y, Makabe K, et al. Humanization of the bispecific epidermal growth factor receptor x CD3 diabody and its efficacy as a potential clinical reagent. Clin Cancer Res. 2006;12:4036–42. doi: 10.1158/1078-0432.CCR-06-0059. [DOI] [PubMed] [Google Scholar]
- 87.Brischwein K, Schlereth B, Guller B, et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–43. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 88.Bruenke J, Barbin K, Kunert S, et al. Effective lysis of lymphoma cells with a stabilised bispecific single-chain Fv antibody against CD19 and FcgammaRIII (CD16) Br J Haematol. 2005;130:218–28. doi: 10.1111/j.1365-2141.2005.05414.x. [DOI] [PubMed] [Google Scholar]
- 89.Marvin JS, Zhu Z. Recombinant approaches to IgG-like bispecific antibodies. Acta Pharmacol Sin. 2005;26:649–58. doi: 10.1111/j.1745-7254.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 90.Biburger M, Weth R, Wels WS. A novel bispecific tetravalent antibody fusion protein to target costimulatory activity for T-cell activation to tumor cells overexpressing ErbB2/HER2. J Mol Biol. 2005;346:1299–311. doi: 10.1016/j.jmb.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 91.Kontermann RE. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol Sin. 2005;26:1–9. doi: 10.1111/j.1745-7254.2005.00008.x. [DOI] [PubMed] [Google Scholar]
- 92.Hudson PJ. Recombinant antibody constructs in cancer therapy. Curr Opin Immunol. 1999;11:548–57. doi: 10.1016/s0952-7915(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 93.Kumar R, Nadig MR, Chauhan A. Positron emission tomography: clinical applications in oncology. Part 1 Expert Rev Anticancer Ther. 2005;5:1079–94. doi: 10.1586/14737140.5.6.1079. [DOI] [PubMed] [Google Scholar]
- 94.Cai W, Olafsen T, Zhang X, et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84. 66 anti-carcinoembryonic antigen diabody. J Nucl Med. 2007;48:304–10. [PubMed] [Google Scholar]
- 95.Sharkey RM, Karacay H, Vallabhajosula S, et al. Molecular imaging with pretargeted ImmunoSPECT and ImmunoPET in a model of metastatic colonic carcinoma. Radiology. 2007 doi: 10.1148/radiol.2462070229. in press. [DOI] [PubMed] [Google Scholar]


